Wheat Yellow Streak Virus N Gene Recombinant Expression Protein, Preparation Method of Polyclonal Antibody and Its Application
A polyclonal antibody and stripe virus technology, which is applied in the field of agricultural biology, can solve problems such as complicated purification procedures, and achieve the effect of simple and convenient operation, speed and practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
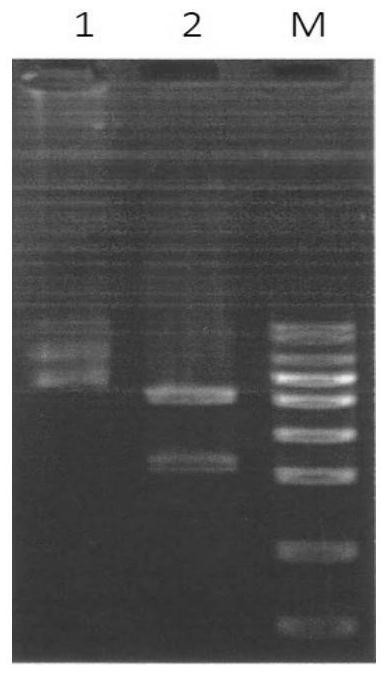
[0032] Embodiment 1. Gene optimization and synthesis, subcloning of wheat yellow streak virus N protein
[0033] According to the full-length gene sequence of WYSV (GenBank accession number MG604920), the protein encoded by ORF1 of the virus, Nucleocapsid protein (N), was analyzed by software, and the N protein gene was found to be 1635 nt in length, encoding 544 amino acids, and the predicted molecular weight and The isoelectric points are 59.8KDa and 8.81, respectively.
[0034] Using the online software SignalP (http: / / www.cbs.dtu.dk / services / SignalP / ) and TMHMMServer v.2.0 (http: / / www.cbs.dtu.dk / services / TMHMM / ) to predict the signal peptide of the protein And the transmembrane region, the analysis found that the N protein of WYSV has no potential signal peptide cleavage site and transmembrane region. Using Optimum Gene TM Codon optimization technology (Nanjing KingScript Technology Co., Ltd.), finally select 365 amino acids (from the 94th to 1188nt of the original prote...
Embodiment 2
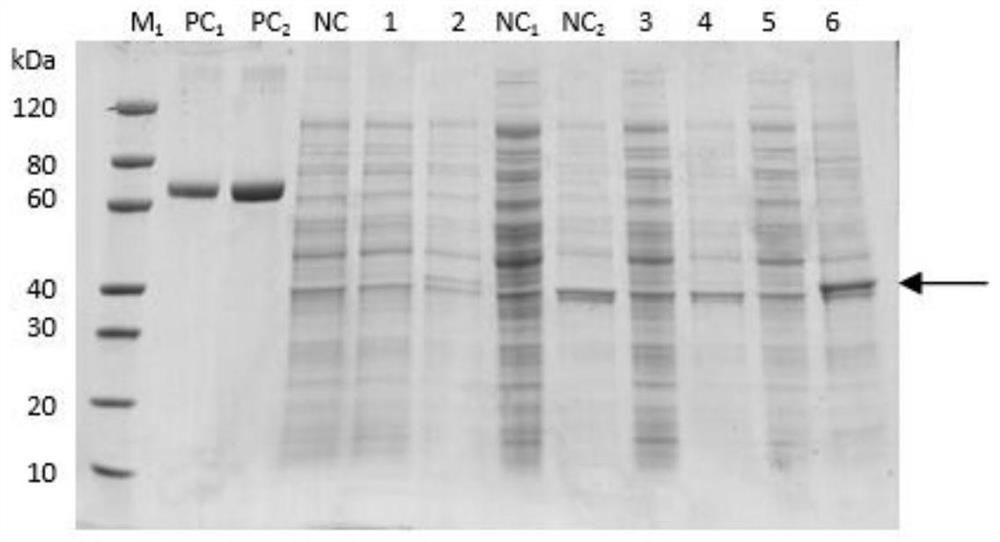
[0035] Embodiment 2. WYSV-N recombinant protein expression and purification
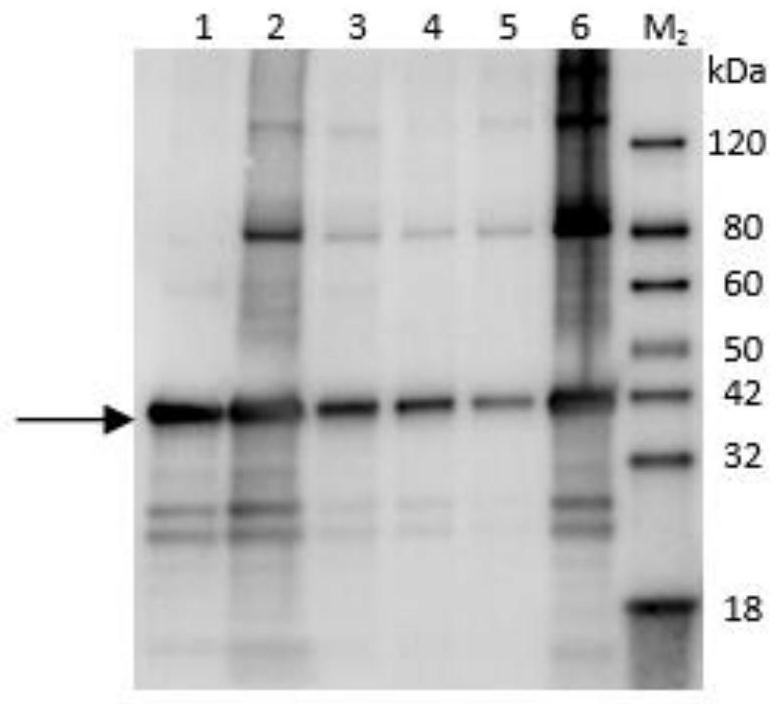
[0036] The recombinant expression plasmid pET-30a-(WYSV-N) was transformed into the BL21(DE3) expression strain by heat shock, and after sequencing verification, the BL21 bacterial solution containing the correct recombinant expression plasmid was inoculated in the culture medium containing 50ug / mL In 4 mL LB liquid medium of kanamycin, culture at 37°C and 200rpm until OD600 is 0.6-0.8, add IPTG to a final concentration of 0.3mmol / L, induce at 15°C for 16h, then continue to induce at 37°C for 4h Collect 1mL of the bacterial liquid, and centrifuge to collect the bacterial cells. Detected by 12% SDS-PAGE gel electrophoresis, the protein expression in the whole bacteria, supernatant and precipitate were detected respectively. It was found that the specific expression of the target protein was detected in the induced sample, and the size was about 42kDa, which was consistent with the expected fusion pro...
Embodiment 3
[0037] The preparation of embodiment 3.N protein antibody
[0038] After the purified soluble protein was emulsified with complete Freund's adjuvant, the New Zealand male rabbits were subcutaneously immunized at multiple points, and the second immunization was carried out 10 days later, and the immunization was boosted every other week, and the booster immunization was emulsified with incomplete Freund's adjuvant. Thigh intramuscular injection method. A small amount of serum was taken 5 days after each immunization, and the titer of the antibody was determined by indirect ELISA. When the titer reached 1:100,000 or more, blood was taken and the serum was separated. Anti-WYSV-N-IgG was purified from antiserum using protein A-Sepharose affinity column, according to 500×2 1 (1000) to 500×2 10 (512 000) serial ratio dilution, the experiment took pre-immune serum as the negative control and PBS buffer as the blank control, the results showed that the titer determined by indirect E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com