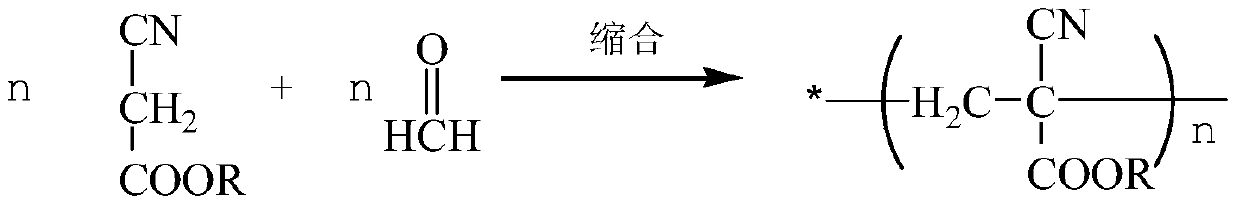
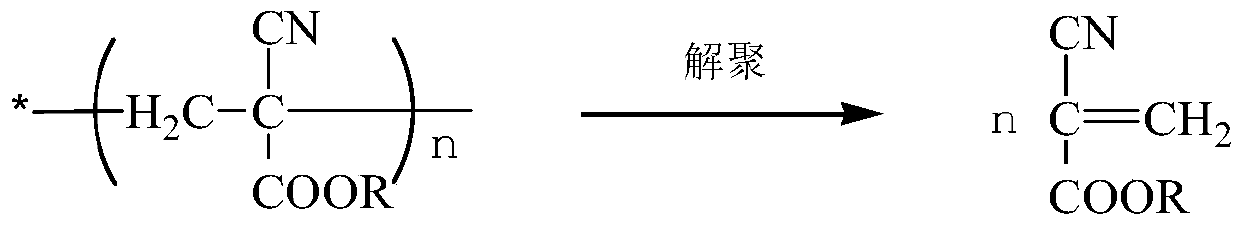
Preparation method of alpha-cyanoacrylate
A technology of cyanoacrylate and cyanoacetate is applied in the field of preparation of α-cyanoacrylate and can solve the problems of low product yield and purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The preparation method of α-ethyl cyanoacrylate monomer:
[0056] ①Mix 300g of ethyl cyanoacetate and solid formaldehyde or formaldehyde solution in dichloroethane or toluene medium, add hexahydropyridine catalyst, control the temperature at 70°C±5°C, and carry out condensation reaction for 2 hours to obtain α-cyanide Oligomers of ethyl acrylate;
[0057] Wherein the feeding amount of ethyl cyanoacetate and formaldehyde aqueous solution (calculated as formaldehyde) is 1.02:1.00, and the addition amount of hexahydropyridine is 0.15% of the weight percentage of ethyl cyanoacetate.
[0058] ②Add 50g of dioctyl phthalate to the oligomer of α-ethyl cyanoacrylate, remove the solvent under normal pressure or reduced pressure until the temperature of the kettle is 140°C, add 8g of phosphorus pentoxide, terephthalate Phenol 4g, cracked under reduced pressure at -0.098Mpa, collected fractions at 150-200°C, and obtained 290g of crude monomer of ethyl α-cyanoacrylate;
[0059] ③T...
Embodiment 2
[0062] The preparation method of n-butyl α-cyanoacrylate:
[0063] Get 500g of the α-ethyl cyanoacrylate monomer prepared in Example 1 and add in a 1000ml flask, add 1% chloroacetic acid, 0.3% hydroquinone and 0.33% concentrated sulfuric acid, add 444g n-butanol, stir Raise the temperature, control the temperature of the kettle to 100-115°C, collect the ethanol fraction at the top of the tower at 76-82°C; Pressure (200-800Pa) to collect fractions with a content > 98% until the temperature at the top of the column drops significantly. 425 g of n-butyl α-cyanoacrylate with a content >99% were obtained.
[0064] Among the present invention, select glacial acetic acid or anhydrous phosphoric acid as the anionic polymerization inhibitor, select methanesulfonic acid or p-toluenesulfonic acid to replace chloroacetic acid and the concentrated sulfuric acid in the present embodiment as the catalyst, as long as the consumption of the anionic polymerization inhibitor is 1-2% of the mol...
Embodiment 3
[0066] The preparation method of n-butyl α-cyanoacrylate:
[0067] Take 500 g of the α-ethyl cyanoacrylate monomer prepared in Example 1 and add it to a 1000 ml flask, add 2% of a mixture of glacial acetic acid and anhydrous phosphoric acid (the molar ratio of glacial acetic acid and anhydrous phosphoric acid is 0.7:1), The mixture of 0.35% hydroquinone and 0.45% concentrated sulfuric acid and p-toluenesulfonic acid (the mol ratio of concentrated sulfuric acid and p-toluenesulfonic acid is 1:1), add 444g n-butanol, stir and heat up, control the temperature of the kettle at 100 -115°C, collect the ethanol fraction at the top of the tower at 76-82°C; increase the temperature of the kettle to 120-140°C, collect n-butanol under normal pressure and water pump decompression (-0.1MPa), and depressurize the oil pump (200-800Pa) Fractions containing >98% were collected until the overhead temperature dropped significantly. 440 g of n-butyl α-cyanoacrylate with a content >99% was obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com