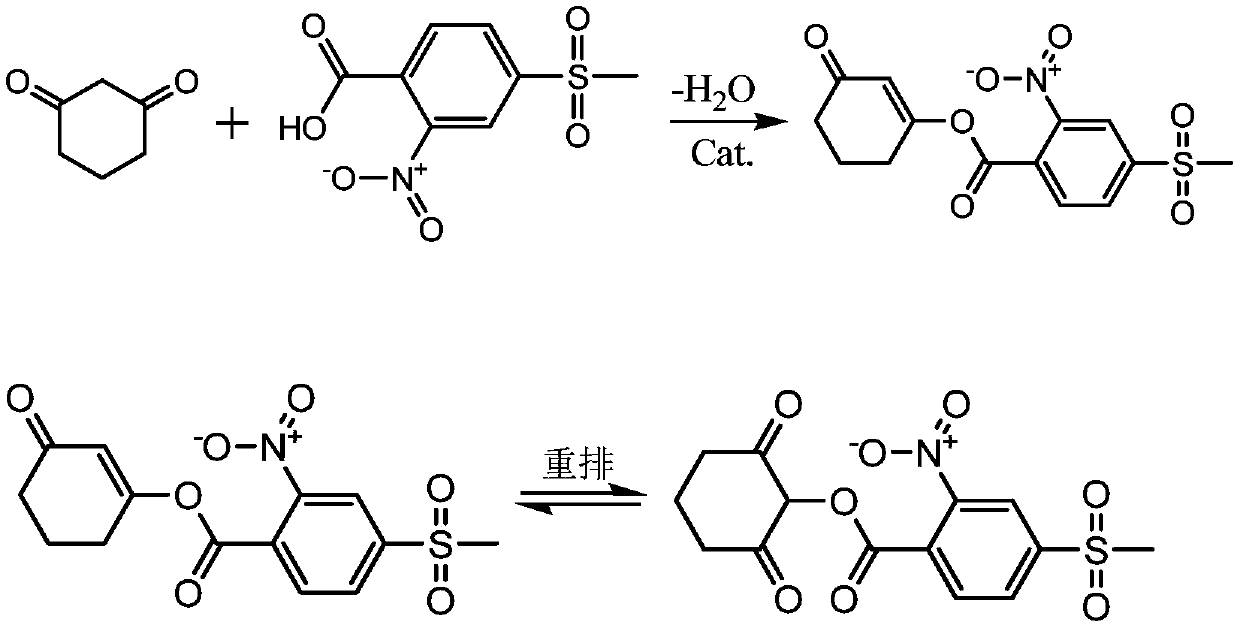
Synthetic process of methyl sulcotrione
A technology of mesotrione and synthesis process, which is applied in the field of synthesis process of mesotrione, can solve the problems of high risk of phosgene, difficulty in separation, complex process, etc., and achieve avoidance of tail gas treatment, reduction of reaction steps, Reduce the effect of wastewater treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Put 13 grams of 2-nitro-4-thiamphenicol benzoic acid and 6 grams of 1,3-cyclohexanedione into a three-necked flask with a stirrer and a condenser tube, then add 250 milliliters of xylene, 0.1 grams of trifluoro Phenylboronic acid and 10 g of dicyclohexylcarbodiimide were heated to reflux at 140-150°C under stirring for condensation reaction, followed by TLC to complete the reaction, rearrangement reaction in acetonitrile, and finally post-treatment to obtain the target compound Mesotrione has a content of 98.0% and a yield of 80.3%.
Embodiment 2
[0019] Put 13 grams of 2-nitro-4-thiamphenicol benzoic acid and 6 grams of 1,3-cyclohexanedione into a three-necked flask with a stirrer and a condenser, add 250 milliliters of xylene, and 0.05 grams of trifluorophenylboronic acid , 10 grams of dicyclohexylcarbodiimide, under stirring and heating to 140-150 ° C reflux. After the completion of the condensation reaction (TLC tracking), it was processed, then rearranged, and finally post-treated to obtain the target compound with a content of 96.6% and a yield of 77.3%.
Embodiment 3
[0021] Put 13 grams of 2-nitro-4-thiamphenicol benzoic acid and 6 grams of 1,3-cyclohexanedione into a three-necked flask with a stirrer and a condenser tube, add 250 milliliters of monochlorobenzene, trifluorophenylboronic acid 0.1 gram, 10 grams of dicyclohexylcarbodiimide, under stirring and heating up to 140-150 ℃ reflux. After the completion of the condensation reaction (TLC tracking), it was processed, and then rearranged, and finally post-treated to obtain the target compound with a content of 93.5% and a yield of 75.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com