Heme ligand mimic and synthesis method thereof
A synthetic method and heme technology, applied in the research field of biological enzyme structure and function simulation, to achieve good water solubility and biocompatibility, low reaction cost, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0052] The present invention will be further described in detail below in conjunction with specific experimental examples.
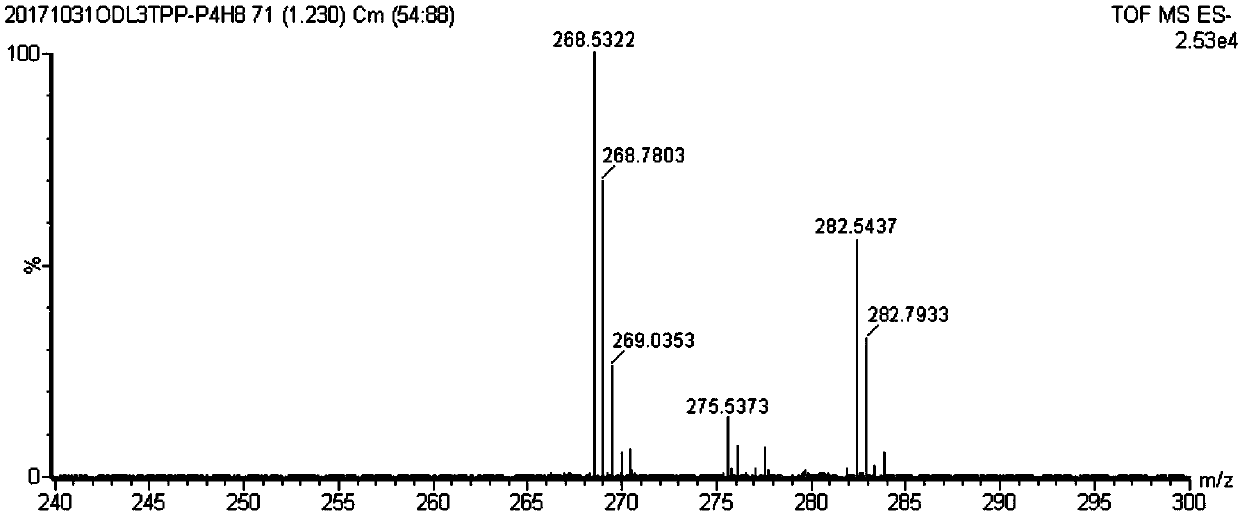
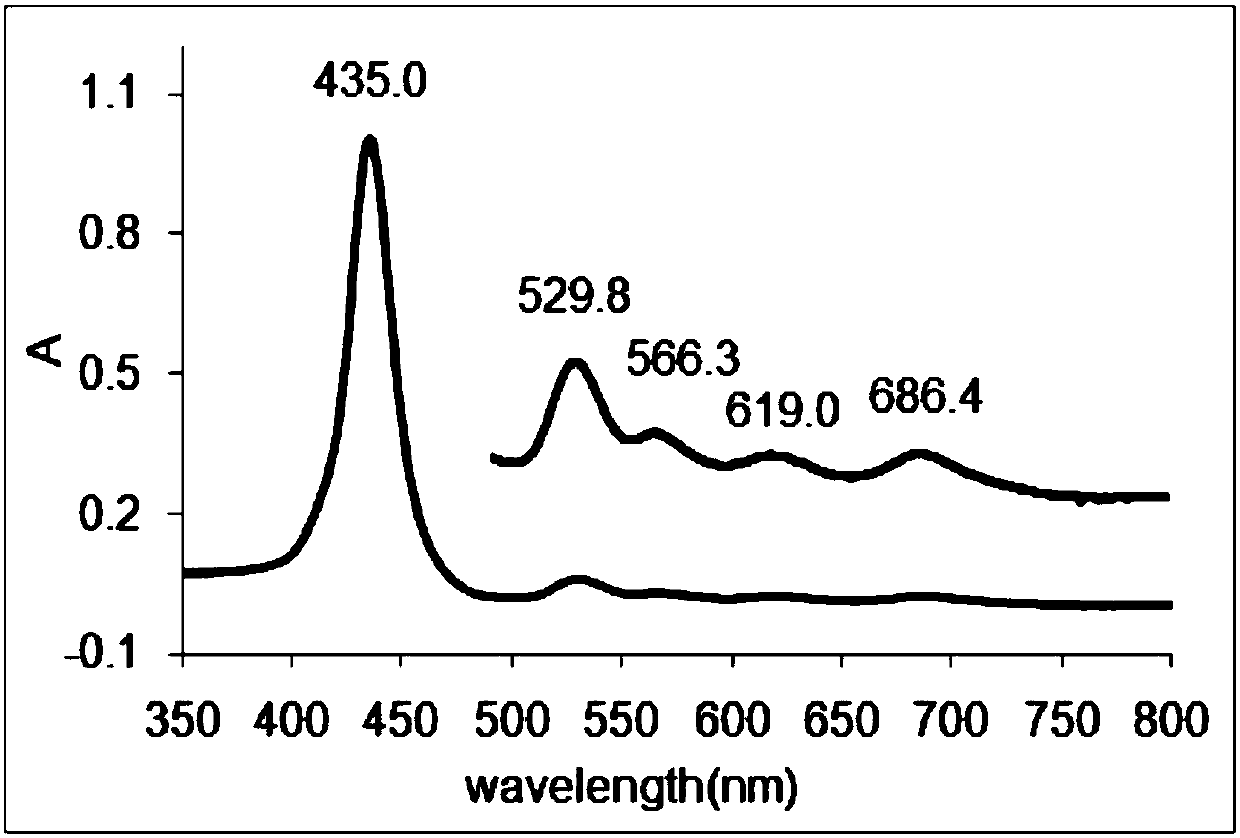
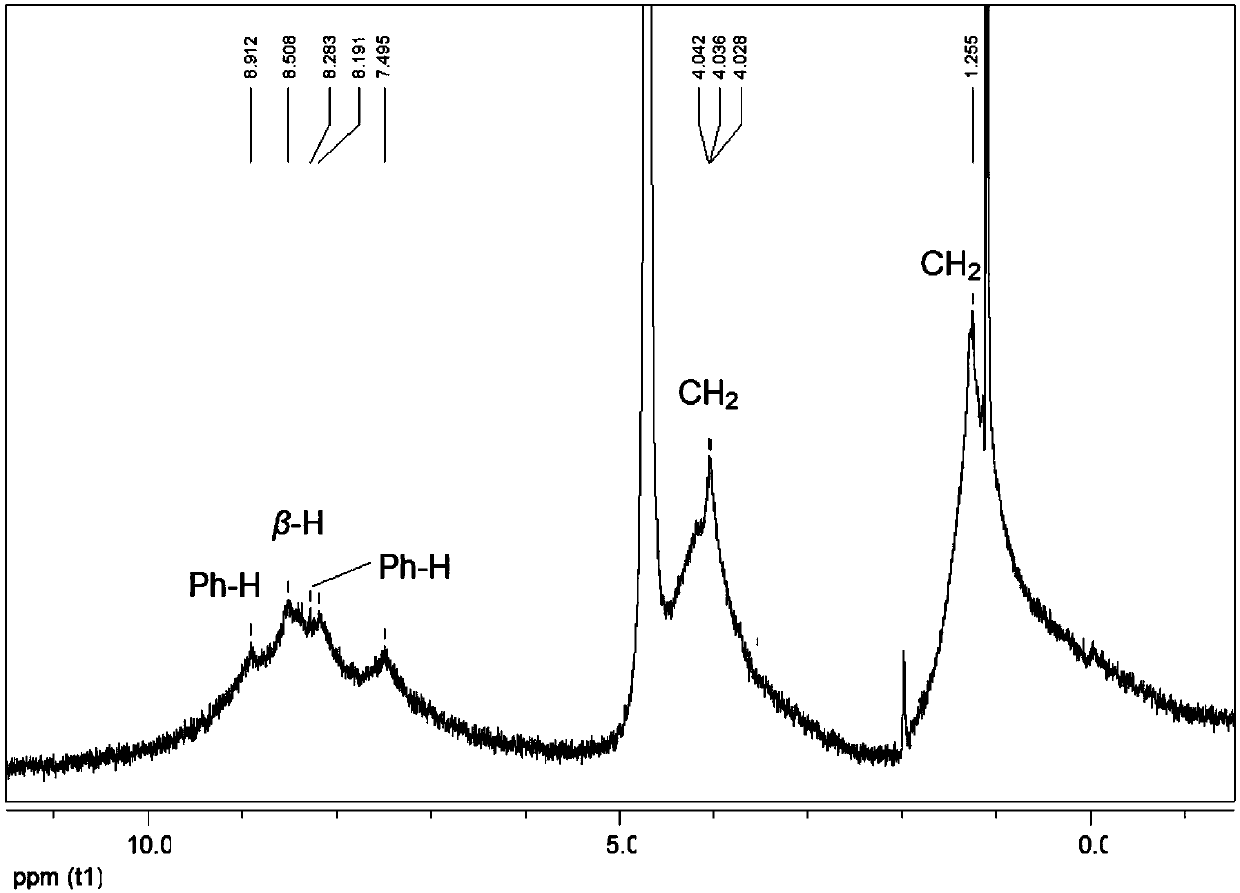
[0053] Phosphorylated water-soluble porphyrin of the present invention, its compound name is 5,10,15,20-tetrakis-(5-phosphophenyl)-5,10-15,20-double bundled porphyrin, and its compound structural formula is as follows: (1) as shown:
[0054]
[0055] Its compound synthetic train of thought route is as shown in formula (5):
[0056]
[0057] As can be seen from the formula (5), its specific synthesis steps are completed in four steps.
[0058] The first step is the bridging of the strapping chain. Use 50ml of DMF as a solvent, heat to a constant temperature of 65°C, add 20g (ie 0.10mol) of 5-bromosalicylaldehyde and 28g (ie 0.2mol, 2 equivalents) of anhydrous potassium carbonate, and keep it for about 10 minutes; 10g of 3-dibromopropane (ie 0.05mol, 0.5 equivalent) was added dropwise to the DMF reaction solution within 20min, continued to keep th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com