Preparation method of Edoxaban tosylate intermediate and intermediate compound
A technology for edoxaban toluenesulfonate and intermediates, which is applied in the field of pharmaceutical preparation, can solve the problems of increased risk, low reaction yield, and lengthy steps, etc., and achieves improved process safety, simple introduction process, and increased production yield. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the preparation of edoxaban tosylate intermediate comprises the following steps,
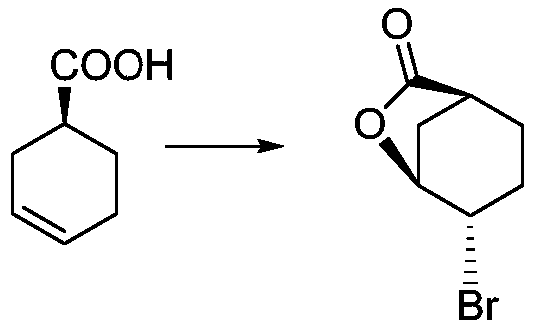
[0022] Step S1: Preparation of (1S,4S,5S)-4-bromo-6-oxabicyclo[3.2.1]oct-7-one, the reaction formula is as follows:
[0023]
[0024] Specific steps: Add 887.7g of N-bromosuccinimide into a 10L reaction flask, add 1L of dichloromethane, and stir to dissolve. 23.44 g of calcium oxide were added. Continue to stir. Control the temperature at about 25°C, dilute 600g of S-cyclohexenecarboxylic acid with 5L of dichloromethane and add to the reaction system. The dropwise addition was completed, and the reaction was completed at this temperature. Filter through celite and wash the filter cake with dichloromethane. The combined filtrates were concentrated to dryness under reduced pressure. Add 3L of distilled water preheated at 35-40°C to the residue, stir to disperse, filter, and rinse with hot water. Repeat hot water beating once. Collect solids. The resulting white soli...
Embodiment 2
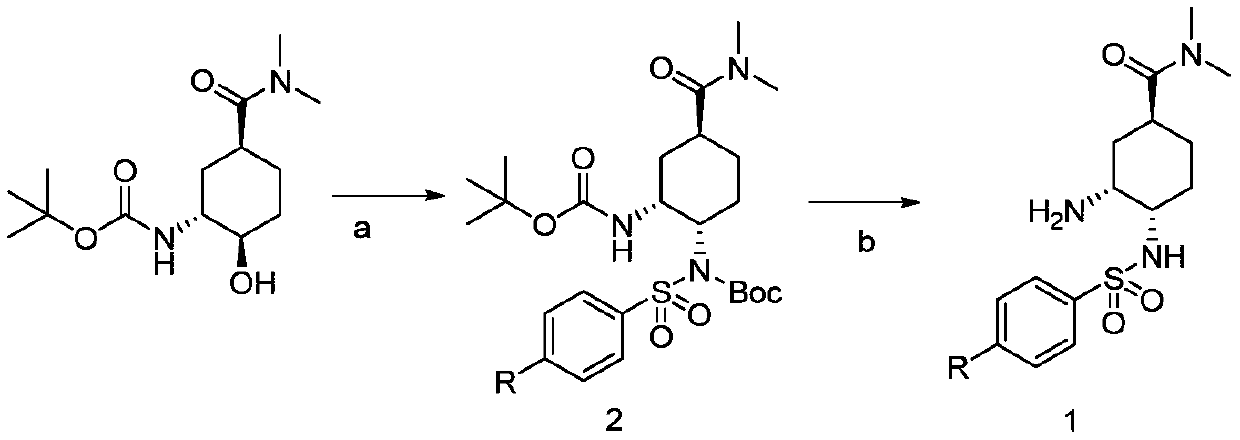
[0031] Embodiment 2: the preparation of edoxaban tosylate intermediate, the difference with embodiment 1 is, in step S3, get the product 48.7g (0.17mol) of step S2 and triphenylphosphine 44.6g (0.17mol ), N-(tert-butoxycarbonyl) p-nitrobenzenesulfonamide 51.4g (0.17mol) was dissolved in 5L of toluene, the reaction solution was cooled to 0°C, and 296g (1.7mol) of diethyl azodicarboxylate was slowly added, React at this temperature for 6 hours, concentrate under reduced pressure to remove the solvent, add 300ml ethyl acetate to the residue, cool down to 0-5°C, stir and crystallize, filter to obtain a white powder solid, (1S,3R,4S)-3- [(tert-butoxycarbonyl)amino]-4-[N-(tert-butoxycarbonyl)p-nitrobenzenesulfonamide]-N,N-dimethylcyclohexanecarboxamide 79.8 g. Yield: 82.2%.
Embodiment 3
[0032] Example 3, the preparation of edoxaban tosylate intermediate, differs from Example 1 in that in step S3, (1S,3R,4S)-3-[(tert-butoxycarbonyl)amino]-4 -[N-(tert-butoxycarbonyl) p-toluenesulfonamide]-N, the preparation of N-dimethylcyclohexanecarboxamide, the reaction formula is as follows:
[0033]
[0034] Concrete steps: Take 23.4g (0.082mol) of the product of step S2, 21.5g (0.082mol) of triphenylphosphine, and 22.2g (0.082mol) of N-(tert-butoxycarbonyl) p-toluenesulfonamide into 450ml of toluene , the reaction solution was cooled to -20°C, and 14.3 g (0.082 mol) of diethyl azodicarboxylate was slowly added, reacted at this temperature for 6h-8h, concentrated under reduced pressure to remove the solvent, and 300ml of ethyl acetate was added to the residue. Cool down to 0-5°C, stir and crystallize, and filter to obtain a white powder solid, (1S,3R,4R)-3-[(tert-butoxycarbonyl)amino]-4-[N-(tert-butoxycarbonyl) Toluenesulfonamide]-N,N-dimethylcyclohexanecarboxamide 30....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com