Method for eliminating unsaturated double bonds in hydrogenated styrene thermoplastic elastomers
A thermoplastic elastomer and hydrogenated styrene technology, which is applied in the field of hydrogenated styrene thermoplastic elastomer modification, can solve the problems of complex process flow, unsafe experimental operation, harsh reaction conditions, etc., and achieves simple reaction process and reaction process. Smooth, mildly conditioned effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
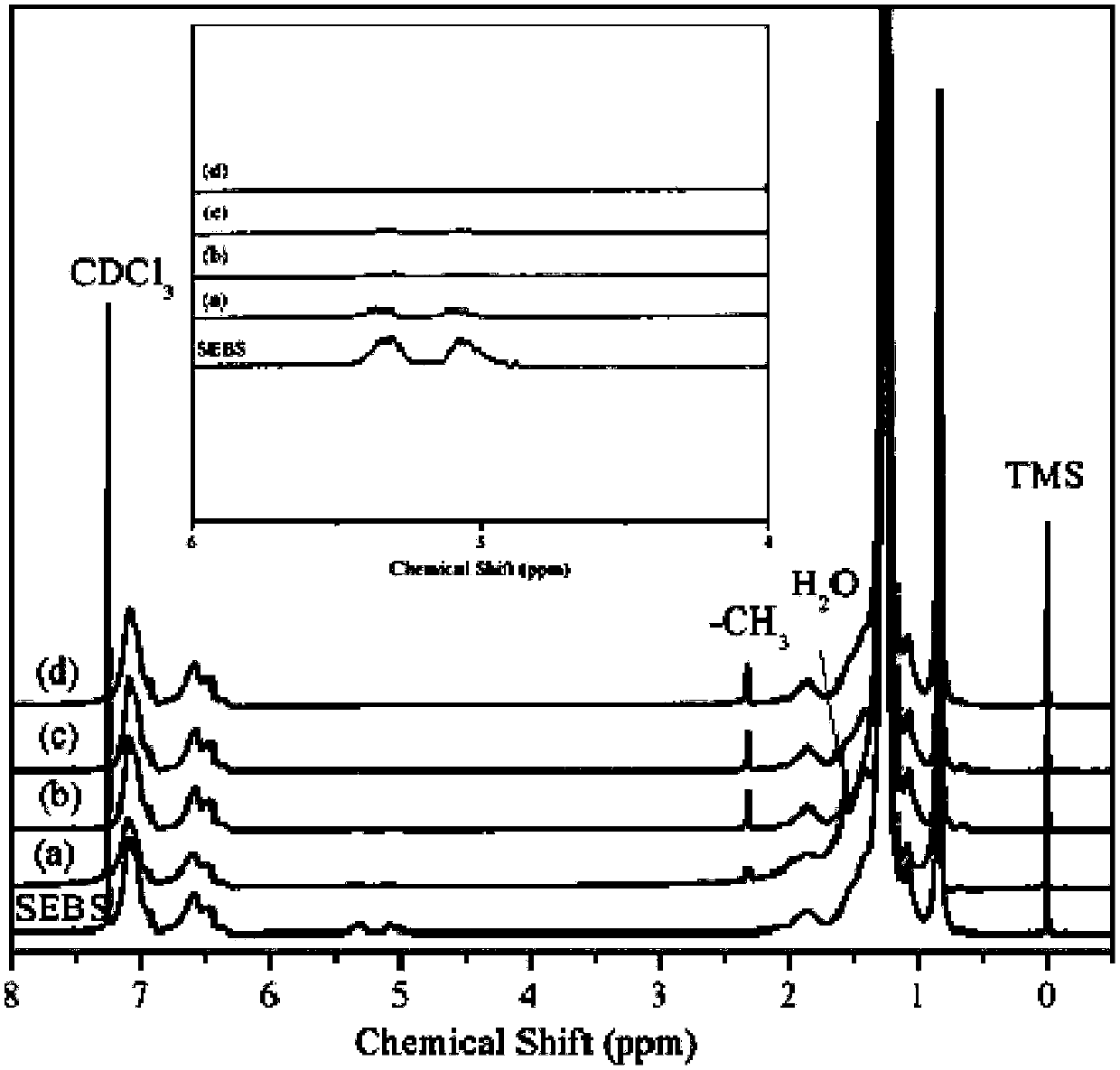
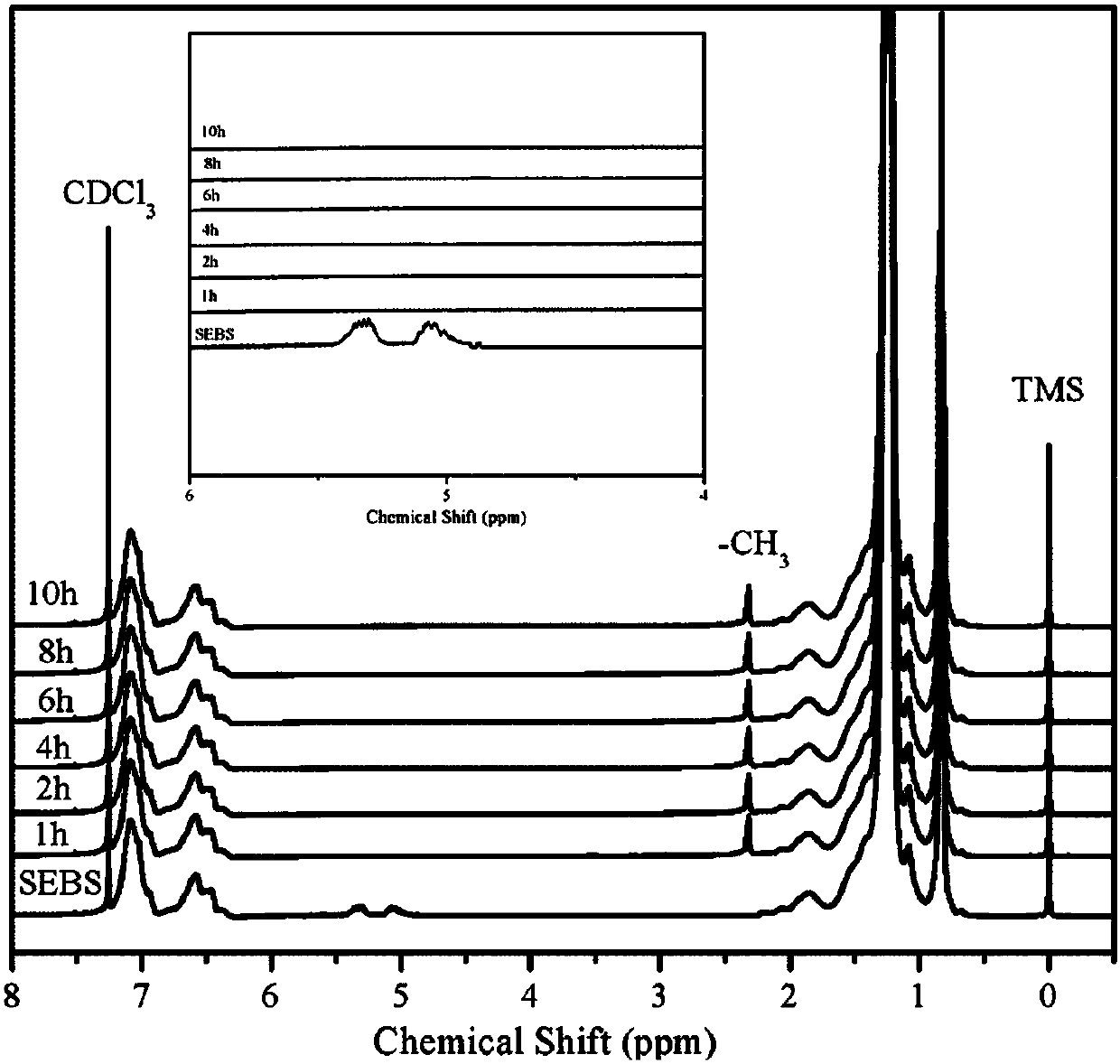
[0035] Weigh 100g of SEBS with unsaturated double bonds, add it into a 1000mL three-necked flask equipped with a stirring device, add 500mL of benzene into it, and after it is fully dissolved, add 4.86g of ferric chloride, the reaction temperature is 25 ° C, and the reaction The time is 8 hours. After the reaction is completed, add 100mL deionized water to terminate the reaction. The reaction solvent is evaporated with water vapor. The initial product is first washed with dilute hydrochloric acid, and then washed with deionized water until neutral, and the initial product is placed at 35°C for vacuum drying. After drying in the oven for 24 hours, the SEBS without unsaturated double bonds can be obtained. According to the detection of nuclear magnetic spectrum, the hydrogen absorption peak on the unsaturated double bonds without 5.0-5.5ppm completely disappears, and at the same time, a new chemical shift of 2.3ppm on toluene is added. the methyl hydrogen absorption peak. Its 10...
Embodiment 2
[0037] Weigh 100g of SEBS with unsaturated double bonds, add it into a 1000mL three-necked flask equipped with a stirring device, add 500mL of toluene into it, and after it is fully dissolved, add 4.86g of ferric chloride, the reaction temperature is 25 °C, and the reaction The time is 5 hours. After the reaction is completed, add 100mL deionized water to terminate the reaction. The reaction solvent is evaporated with water vapor. The initial product is first washed with dilute hydrochloric acid, and then washed with deionized water until neutral, and the initial product is placed at 35°C for vacuum drying. After drying in the oven for 24 hours, the SEBS without unsaturated double bonds can be obtained. According to the detection of nuclear magnetic spectrum, the hydrogen absorption peak on the unsaturated double bonds without 5.0-5.5ppm completely disappears, and at the same time, a new chemical shift of 2.3ppm on toluene is added. the methyl hydrogen absorption peak. Its 10%...
Embodiment 3
[0039] Weigh 100g of SEBS with unsaturated double bonds, put it into a 1000mL three-necked flask equipped with a stirring device, add 500mL of n-propylbenzene into it, and after it is fully dissolved, add 4.86g of ferric chloride, and the reaction temperature is 25°C , the reaction time is 5h. After the reaction is completed, add 100mL deionized water to terminate the reaction. The reaction solvent is evaporated with water vapor. The initial product is washed with dilute hydrochloric acid, and then washed with deionized water until neutral. Dry in a vacuum oven for 24 hours to obtain SEBS without unsaturated double bonds. According to the detection of nuclear magnetic spectrum, there is no unsaturated double bond of 5.0-5.5ppm, and the hydrogen absorption peak completely disappears. At the same time, a new chemical shift of 2.3ppm is added The methyl hydrogen absorption peak on toluene. Its 10% toluene solution viscosity at 25°C is 312mpa.s.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com