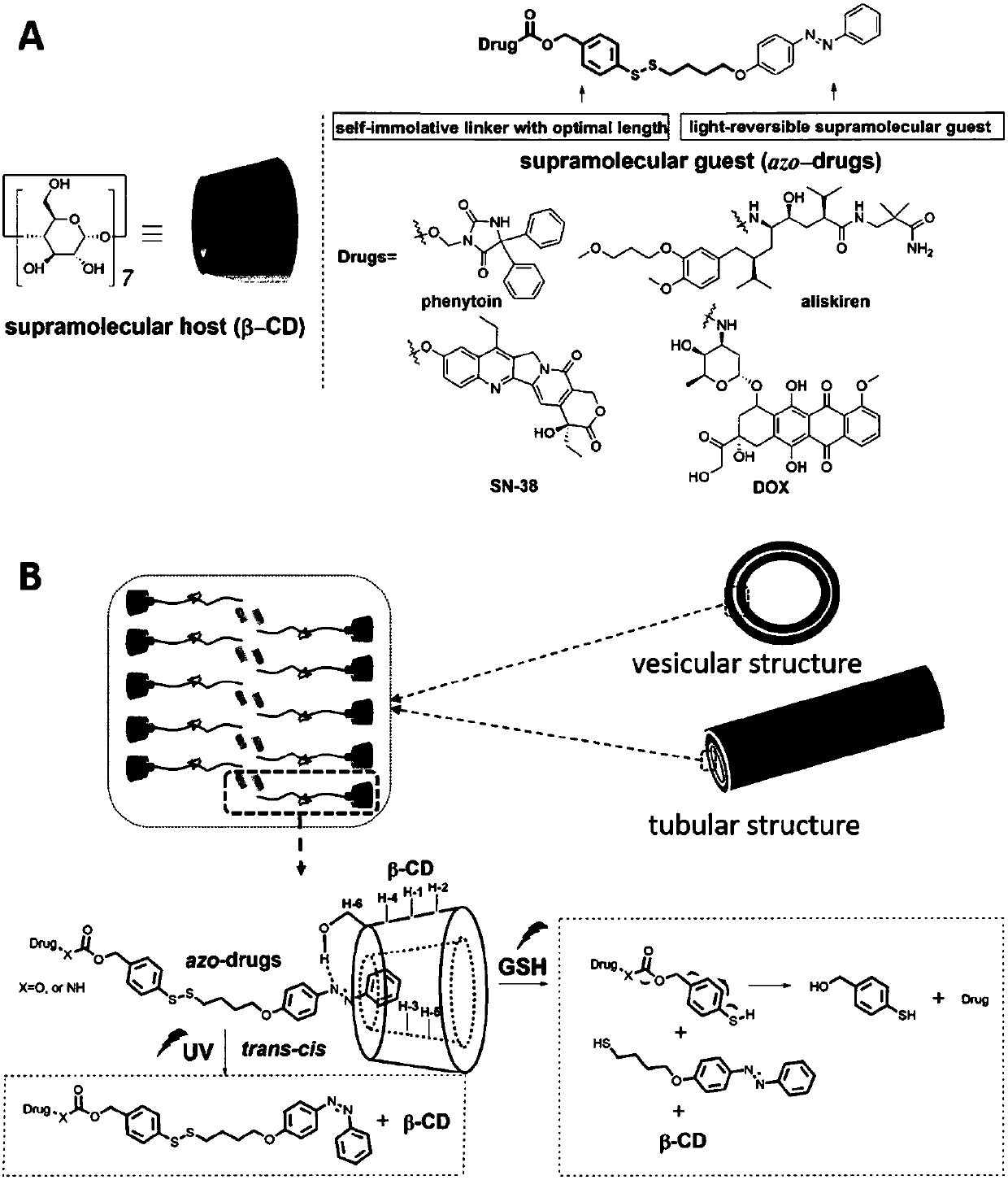
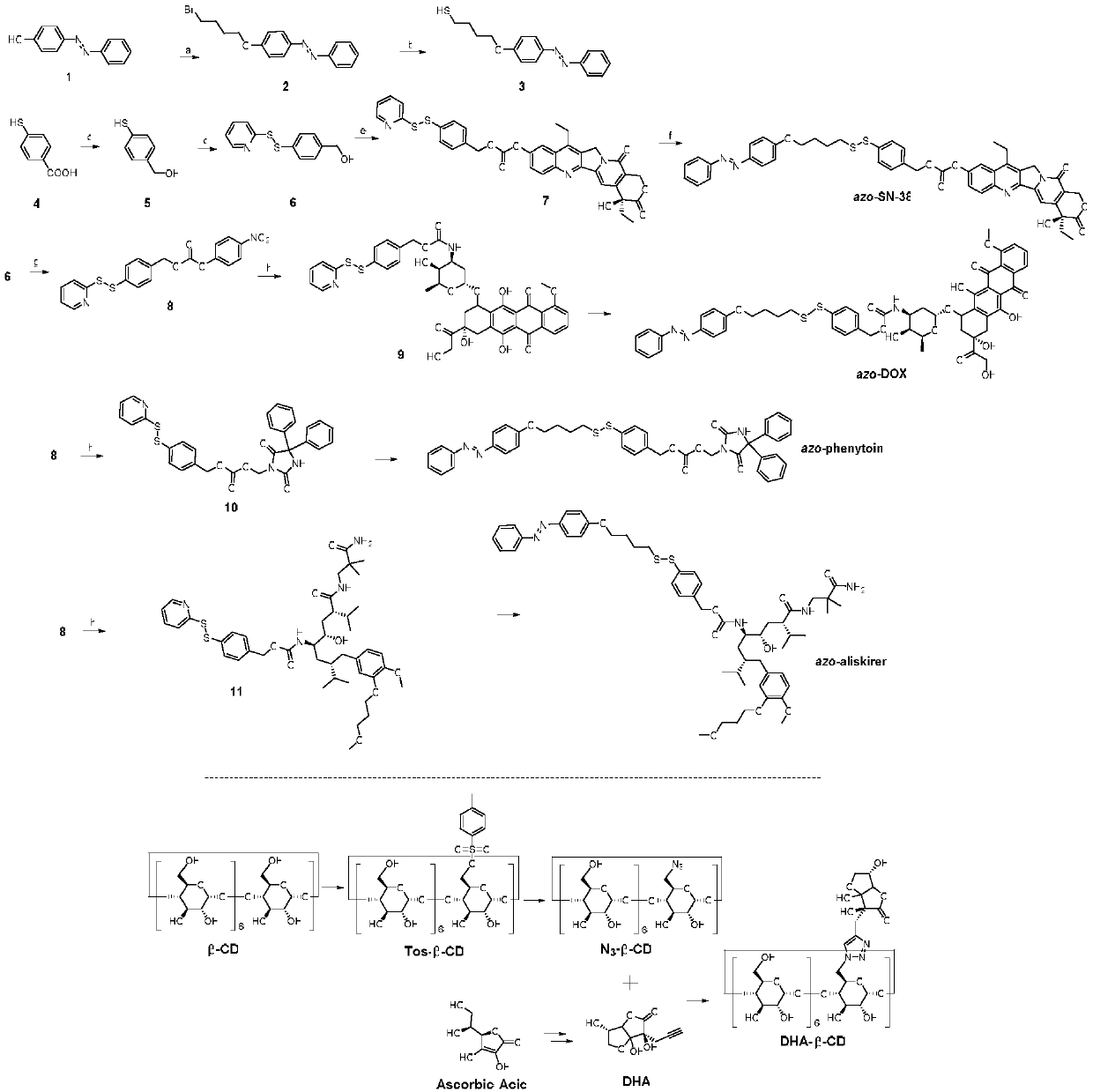
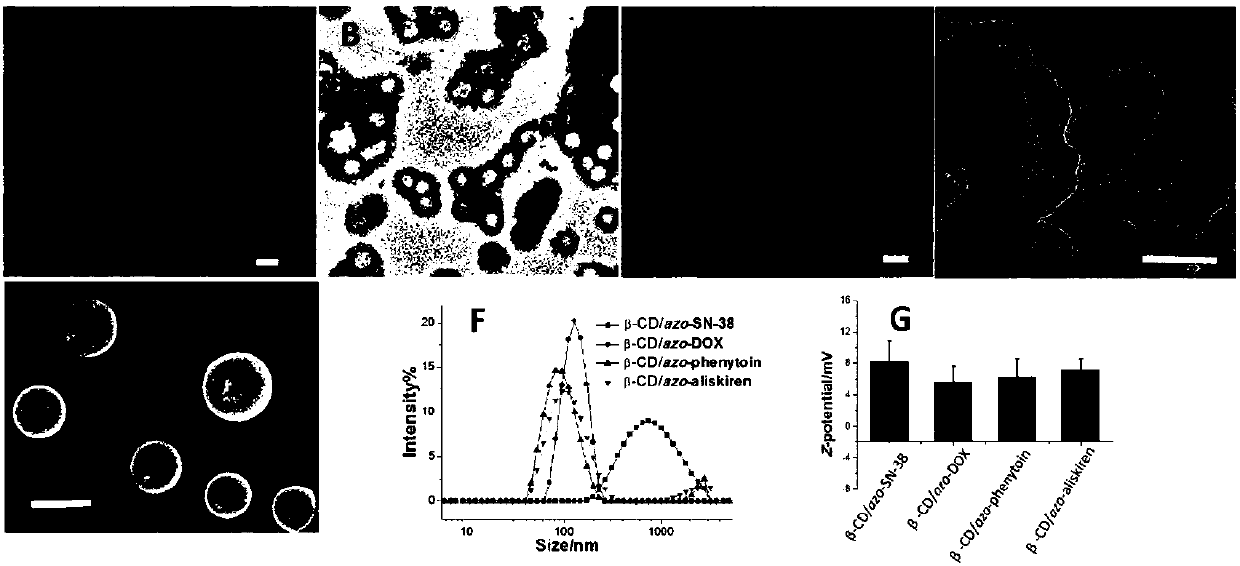
Supramolecular surfactant constructed by cyclodextrin and hydrophobic medicine and preparation method thereof
A technology of surfactants and hydrophobic drugs, applied in drug combinations, pharmaceutical formulations, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
[0056] Synthesis of compound 2: p-hydroxyazobenzene (1.98g, 10mmol, 1eq), 1,4-dibromobutane (7.1mL, 60mmol, 6eq) and solid potassium hydroxide (1.12g, 20mmol, 2eq) were dissolved In ethanol (150 mL), reflux under nitrogen atmosphere for 12 hours, cool to room temperature and remove the solvent under reduced pressure. The residue was resuspended in dichloromethane (100 mL), and the solid was filtered off to obtain crude compound 2, which was purified by flash preparative column chromatography (ethyl acetate:petroleum ether, 1:10 to 1:5 v / v) to obtain brown granular pure product (1.5g, 45%).
[0057] Rf=0.9 (ethyl acetate / petroleum ether, 1 / 8v / v); 1 H NMR (400MHz, CDCl 3 ,δ,ppm):7.92(d,J=8.8Hz,2H,H-4,5),7.88(d,J=7.6Hz,2H,H-6,7),7.51(t,J=7.6Hz ,2H,H-2,3),7.45(dd,J1=6.0Hz,J2=7.6Hz,1H,H-1),7.01(d,J=8.8Hz,2H,H-8,9),4.09 (t,J=6.4Hz,2H,H-10,11),3.51(t,J=6.4Hz,2H,H-16,17),2.09(m,2H,H-14,15),2.00( m,2H,H-12,13); 13 CNMR (150MHz, CDCl 3 ,δ,ppm):160.8,152.1,146.3,129.8(...
Embodiment 2
[0059]
[0060] Synthesis of Compound 3: Compound 2 (0.19g, 0.57mmol, 1eq) and thiourea (0.22g, 2.89mmol, 5eq) were dissolved in ethanol (10mL), and heated to reflux for 12 hours. After cooling down to room temperature, potassium hydroxide (0.19g, 3.47mmol) was dissolved in water (10mL), added to the above solution, heated to reflux under nitrogen atmosphere for 3 hours to room temperature, and the pH was adjusted to 1.0 with hydrochloric acid (1N). Extract with diethyl ether (30 mL×3). The combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, ether was removed under vacuum, and purified by flash preparative column chromatography (ethyl acetate:petroleum ether, 1:5v / v) to obtain pure brown oily product (0.12g, 75%).
[0061] Rf=0.8 (ethyl acetate:petroleum ether, 1:5v / v); 1 H NMR (400MHz, CDCl 3 ,δ,ppm):7.92(d,J=8.8Hz,2H,H-4,5),7.88(d,J=7.6Hz,2H,H-6,7),7.51(t,J=7.6Hz ,2H,H-2,3),7.45(dd,J1=6.0Hz,J2=7.6Hz,1H,H-1),7.00(d,J=8.8Hz,2H,...
Embodiment 3
[0063]
[0064] Compounds 5-8 were synthesized according to previous reports.
[0065]
[0066] Synthesis of compound SN-38-azophenyl group coupler: Compound 7 (180mg, 0.27mmol, 1.5eq) was dissolved in acetic acid / ethanol mixture (1 / 20v / v, 5mL, saturated with nitrogen gas at room temperature 3 minutes), compound 3 (51mg, 0.18mmol, 1eq) was dissolved in acetic acid / ethanol mixture (1 / 20v / v, 5mL, saturated with nitrogen bubbling for 3 minutes), and slowly dropped to above solution, and reacted at room temperature for 12 hours. After removal of the solvent under reduced pressure, purification by flash preparative column chromatography (ethyl acetate: dichloromethane, 1:2 v / v) gave the pure product (55 mg, 36%) as dark yellow granules.
[0067] Rf=0.7 (ethyl acetate: dichloromethane, 1:1 v / v); 1 H NMR (400MHz, CDCl 3 ,δ,ppm):8.22(d,J=9.6Hz,1H,H-25),7.88-7.81(m,5H,H-4,5,6,7,24),7.65-7.59(m,4H ,H-18,19,26,34),7.55-7.53(m,1H,H-1),7.48-7.40(m,4H,H-2,3,20,21),6.95(d,J= 8.8Hz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com