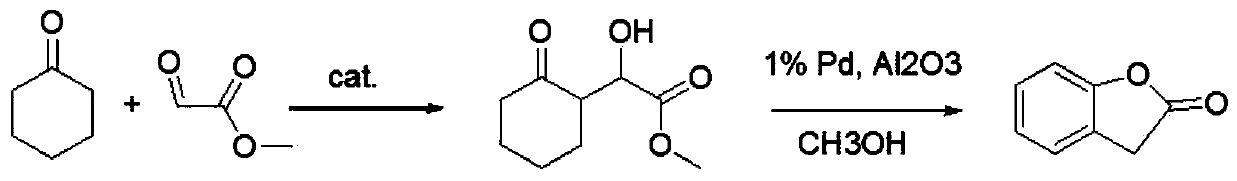
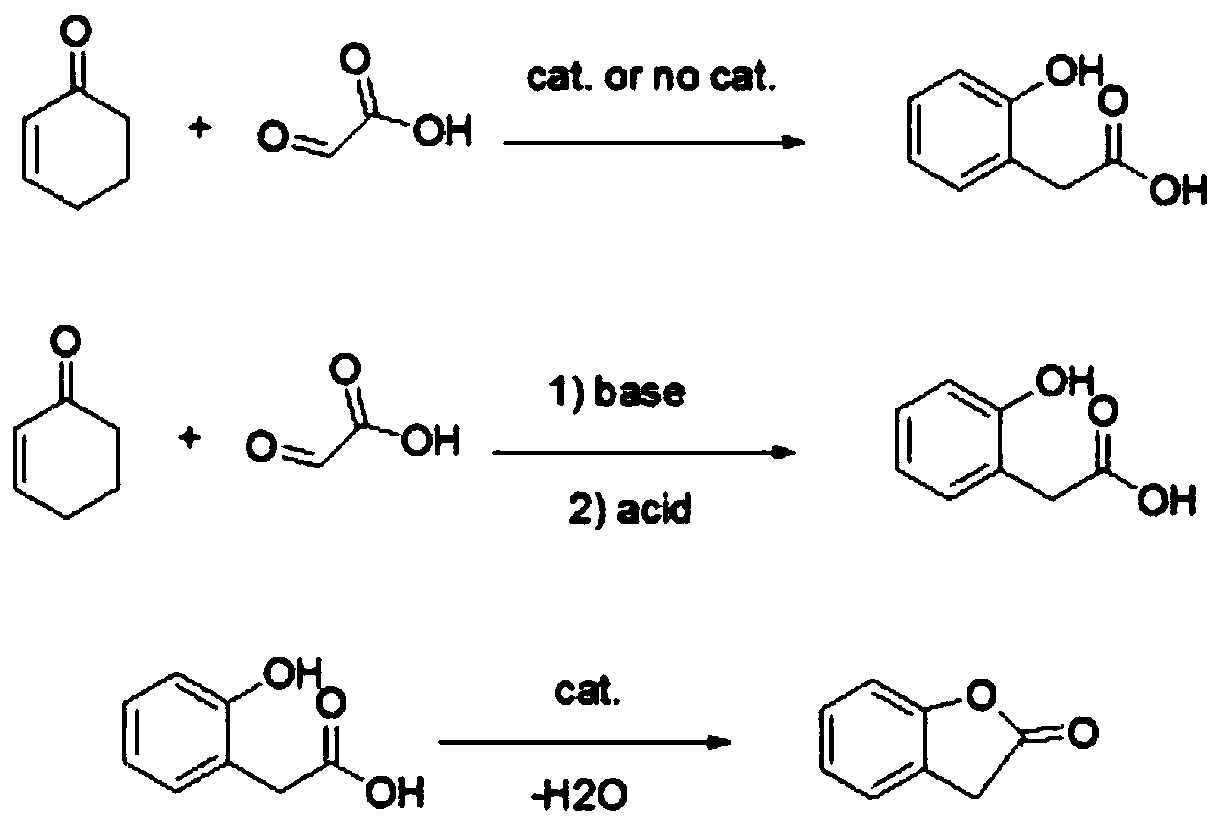
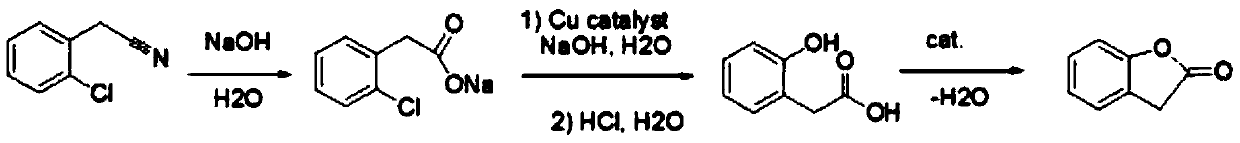Preparation method of 2-coumaranone
A technology for benzofuran and enone, which is applied in the field of preparation of benzofuran-2--one, can solve the problems of large environmental pollution, short synthesis route, difficult operation and the like, and achieves high yield, short synthesis route and low cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation method of benzofuran-2-(3H)-one in this embodiment, the specific steps are:
[0030] 1. Preparation of o-hydroxyphenylacetic acid under acidic conditions
[0031] Add 370g of cyclohex-2-enone and 543g of 50% glyoxylic acid aqueous solution in a 2000ml three-necked round bottom flask with a thermometer and a reflux condenser, and heat to an internal temperature of 105°C for 15 hours under stirring. Cool to room temperature after the reaction, change reflux to distillation, distill off solvent water and excess cyclohex-2-enone under reduced pressure, add 300ml of water, heat and stir for 1 hour, cool to crystallize, filter, and dry to obtain 339g of light yellow solid , yield 79%.
[0032] 2. Preparation of benzofuran-2-(3H)-one
[0033] Add 220g of o-hydroxyphenylacetic acid, 1000ml of anhydrous toluene and 22g of solid sulfonic acid (produced by Kairui Environmental Technology Co., Ltd.) in a 2000ml three-necked round bottom flask with a water separato...
Embodiment 2
[0035] The preparation method of benzofuran-2-(3H)-one in this embodiment, the specific steps are:
[0036] 1. Preparation of o-hydroxyphenylacetic acid under acidic conditions
[0037] Add 370g of cyclohex-2-enone, 543g of 50% glyoxylic acid aqueous solution and 400ml of ethylene glycol dimethyl ether into a 2000ml three-neck round bottom flask with a thermometer and a condenser tube; under stirring, heat to an internal temperature of 120 React at ℃ for 10 hours; cool to room temperature, change reflux to distillation, distill off solvent water, dimethyl ether and excess cyclohex-2-enone by distillation under reduced pressure, add 300ml of water, heat and stir for 2 hours, After cooling and crystallization, it was filtered to obtain 370.6 g of a light yellow solid with a yield of 86.3%.
[0038] 2. Preparation of benzofuran-2-(3H)-one
[0039]Add 220g of o-hydroxyphenylacetic acid, 1000ml of toluene and 5g of potassium hydrogensulfate into a 2000ml three-necked round bottom...
Embodiment 3
[0041] The preparation method of benzofuran-2-(3H)-one in this embodiment, the specific steps are:
[0042] 1. Preparation of o-hydroxyphenylacetic acid under acidic conditions
[0043] Add 370g of cyclohex-2-enone, 543g of 50% glyoxylic acid aqueous solution, 400ml of ethylene glycol dimethyl ether and 20g of solid sulfonic acid catalyst in a 2000ml three-necked round bottom flask with a thermometer and a condenser, and stir , heated to an internal temperature of 130°C for 8 hours. Cool to room temperature, filter off the catalyst, change reflux to distillation, distill off solvent water, ethylene glycol dimethyl ether and recover excess cyclohex-2-enone by distillation under reduced pressure, add 300ml of water, heat and stir for 1 hour, After cooling and crystallization, filter to obtain 411.4 g of light yellow solid with a yield of 95.8%.
[0044] 2. Preparation of benzofuran-2-(3H)-one
[0045] Add 220g o-hydroxyphenylacetic acid, 1000ml toluene and 20g solid phosphoni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com