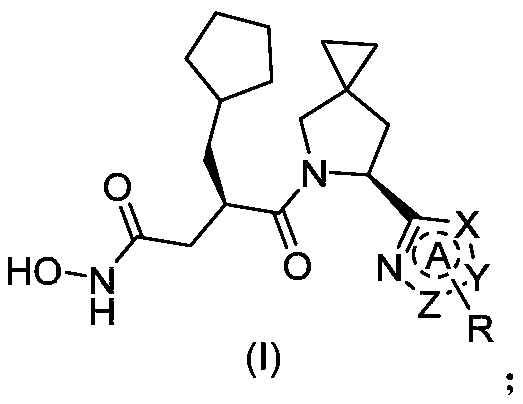
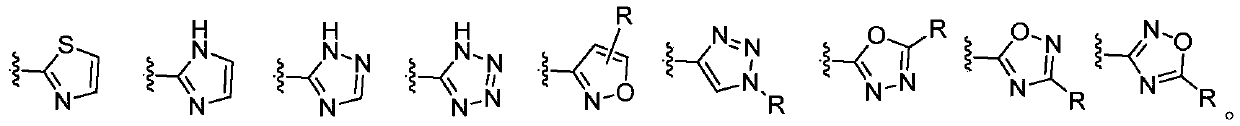
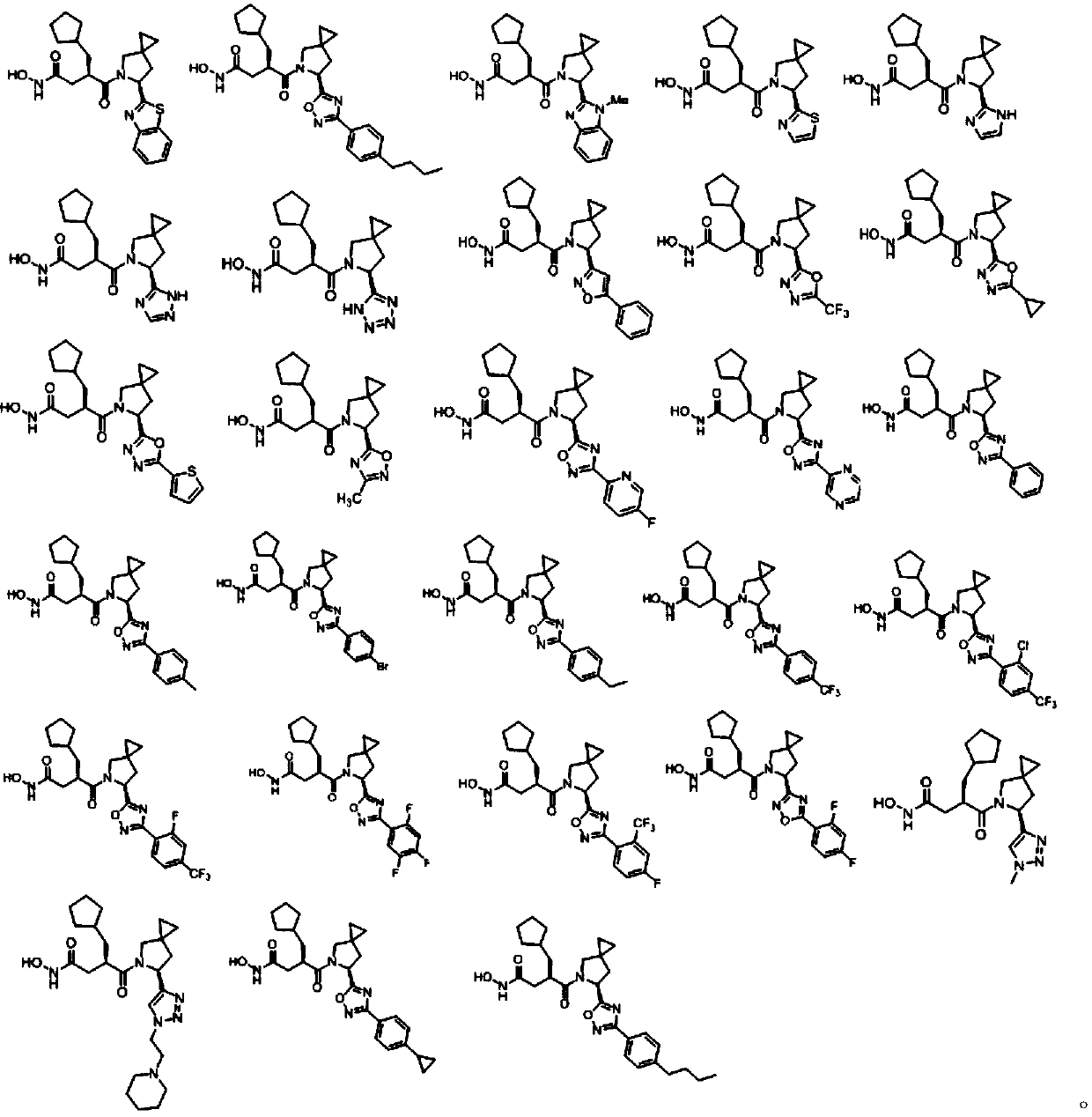
Heterocyclic peptide deformylase inhibitor and preparation method and application thereof
A peptide deformylase and inhibitor technology, applied in the field of heterocyclic peptide deformylase inhibitors and their preparation, can solve the problem of late start of research, and achieve the effect of good inhibitory effect, novel structure and inhibition of proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment 1 compound 1 to 3
[0049] Concrete preparation route is as follows:
[0050]
[0051] 1. The preparation method of intermediate A:
[0052] Step 1: Carboxylic acid (1 equiv), anthranilic acid (1.3 equiv), N,N-diisopropylethylamine (2 equiv) and propylphosphonic anhydride (1 equiv) were added in a sealed tube. Put the sealed tube into a microwave reactor and react at 100°C for 10 minutes. After the reaction was completed, water was added to dilute the reaction solution, alkalized with saturated sodium bicarbonate solution, ethyl acetate was added, the upper organic phase was separated, and the aqueous phase was extracted once. The organic phases were combined, washed successively with saturated sodium bicarbonate solution and saturated sodium chloride solution, dried over anhydrous sodium sulfate, concentrated, and purified by column chromatography to obtain a pure product.
[0053] Step 2: The product obtained in Step 1 was dissolved...
Embodiment 2
[0074] The preparation of embodiment 2 compound 4 to 7
[0075] The preparation route is as follows:
[0076]
[0077] 1. The preparation method of intermediate D:
[0078] Step 1: Weigh carboxylic acid (8.3g, 30mmol) into a reaction flask, dissolve in tetrahydrofuran, and stir in an ice-water bath. HOBt (1.5 eq) and DIPEA (3 eq) were added, EDCI (1.5 eq) was added after reacting at 0°C for 15 minutes, and ammonia (1.1 eq) was added half an hour later. After the raw materials have reacted, the reaction solution is spin-dried. Water and ethyl acetate were added to extract. The aqueous phase was back-extracted once more, and the organic phases were combined. The organic phase was washed once with saturated sodium bicarbonate solution and saturated brine once, then dried over anhydrous sodium sulfate, filtered and concentrated to obtain an oily product.
[0079] Step 2: Weigh the reaction product of the previous step (590 mg, 2.1 mmol) into a reaction flask, dissolve it i...
Embodiment 3
[0112] The preparation of embodiment 3 compound 8
[0113] The preparation route of compound 8 is as follows:
[0114]
[0115] Preparation of Intermediate H:
[0116] Step 1: Dissolve aldehyde (1 equivalent) in 15ml of methanol and stir under ice-water bath. Solid sodium bicarbonate (1.2 eq) and hydroxylamine hydrochloride (1.2 eq) were added, allowed to warm to room temperature, and stirred overnight. After the reaction was complete, the methanol was spin-dried, ethyl acetate was added, the organic phase was washed once with saturated brine, dried with anhydrous sodium sulfate, filtered and spin-dried to obtain the product.
[0117] Step 2: Put the reaction product of the previous step (1 equivalent) in a reaction bottle, dissolve it in 10 mL of DMF, stir in an ice-water bath, first add N-chlorosuccinimide (0.2 equivalent), and remove the ice-water bath after half an hour of reaction , add the remaining NCS (0.8 eq) and react at room temperature for 10 hours. Water an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com