Preparation method of polylactone
A technology of polylactone and ester compounds, which is applied in the field of polylactone preparation, can solve problems such as the inability to realize lactide solution polymerization, and achieve the effects of large commercial application potential, low loading capacity, and narrow molecular weight distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
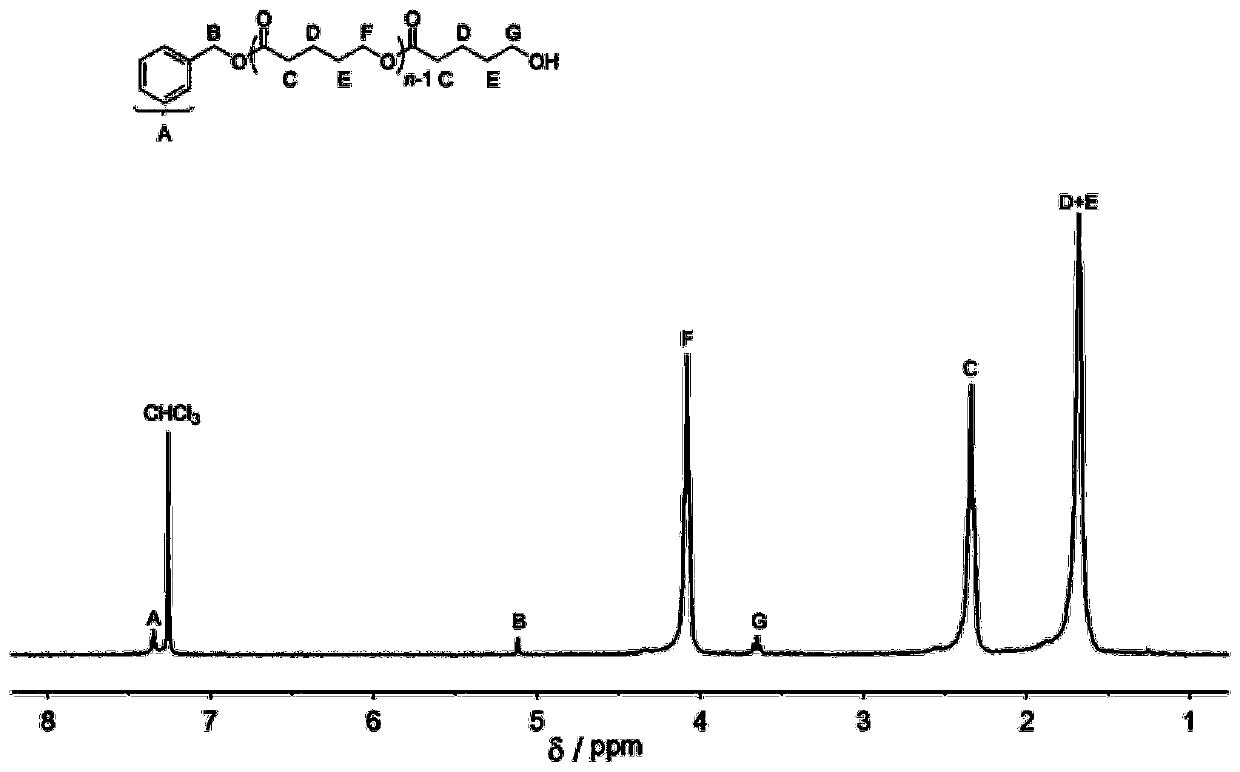
[0047] Add carbocation salt 1 (8.9mg, 0.05mmol, 1.0equiv), benzyl alcohol (5.2μL, 0.05mmol, 1.0equiv) and δ-valerolactone (150mg, 1.5mmol, 30equiv) into the reaction flask, and use 1.5 mL of dichloromethane was dissolved, and under the protection of argon, the reaction was stirred at room temperature for 2 hours. Add triethylamine to terminate the reaction, pour the reactant into methanol after concentration, filter the precipitate and dry to constant weight to obtain 102mg white solid with a conversion rate of 95%, and the number average molecular weight Mn of polyδ-valerolactone is 4.3kg mol -1 , the dispersion PDI is 1.07; its hydrogen spectrum is as follows figure 1 Shown: 1 H NMR (300MHz, CDCl 3 ):δ(ppm)1.68(m,2H×n,(–CH 2 CH 2 CH 2 O–)n),1.70(m,2H×n,(–COCH 2 –CH 2 CH 2 –)n), 2.34(t, 2H×n, J=6.8Hz, (–OCOCH 2 CH 2 –)n),3.65(t,2H,J=6.1Hz,–CH 2 CH 2 OH), 4.08(t, 2H×n, J=5.5Hz, (–CH 2 CH 2 O-)n),5.12(s,2H,ArCH 2 O), 7.32–7.39 (m, 5H, aromatic).
Embodiment 2
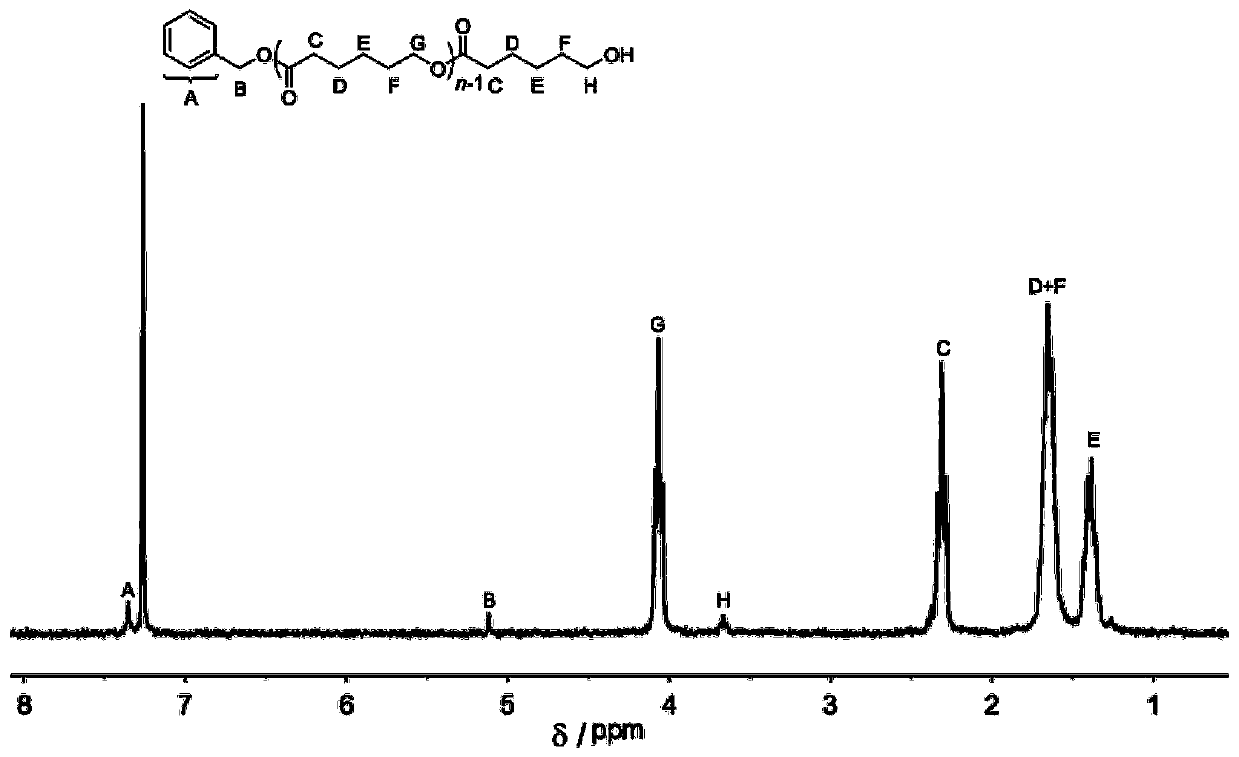
[0049] Carbocation salt 4 (17.2mg, 0.05mmol, 1.0equiv), benzyl alcohol (5.2μL, 0.05mmol, 1.0equiv) and trimethylene carbonate (153mg, 1.5mmol, 30equiv) were added to the reaction flask, under air Under the conditions, the bulk reaction was stirred at 50° C. for 2 hours. Add triethylamine to terminate the reaction, concentrate the reactant and pour it into methanol, filter the precipitate and dry to constant weight to obtain 94.5 mg of white solid with a conversion rate of 92%. The number average molecular weight Mn of polytrimethylene carbonate is 3.5 kg mol -1 ; Dispersion PDI is 1.12; its hydrogen spectrum data such as image 3 Shown: 1 H NMR(300MHz,Chloroform-d)δ(ppm),7.16~7.30(m,5H,aromatic),4.13~4.31(m,2H,(ArCH 2 CH 2 CH 2 -); m,4H×n,(-OCH 2 CH 2 CH 2 O-)n),3.73(t,2H,-CH 2 OH), 2.71(t,2H,ArCH 2 -),2.00~2.08(m,2H,(ArCH 2 CH 2 -); m,2H×n,(-OCH 2 CH 2 -)), 1.91(q,2H,-CH 2 CH 2 OH).
Embodiment 3
[0051] Add carbocation salt 2 (26.8mg, 0.05mmol, 1.0equiv), benzyl alcohol (5.2μL, 0.05mmol, 1.0equiv) and ε-caprolactone (171mg, 1.5mmol, 30equiv) into the reaction flask, and use 1.5 mL of toluene was dissolved, and under the protection of argon, the reaction was stirred at room temperature for 6 hours. Triethylamine was added to terminate the reaction, the reactant was concentrated and poured into methanol, the precipitate was filtered and dried to constant weight to obtain 94.5 mg of white solid with a conversion rate of 53%. From the MALDI-ToF MS spectrum of polycaprolactone ( Figure 10 ) data, there is only one ion peak cluster with Poisson distribution in the spectrogram, and the average difference between adjacent ion peaks is 114m / z, which is exactly the molecular weight of the ε-caprolactone unit, which also has the adjacent mass difference of 114m / z Other series of ion peaks are different signals due to the addition of other types of ions. The molar mass of two hi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com