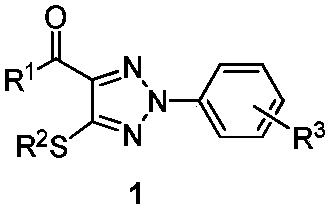
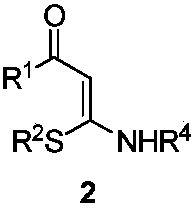
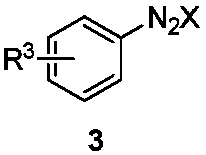
1,2,3-Triazole derivative as well as synthesis and application thereof
A technology of triazole derivatives and trifluoromethyl, which is applied in the field of 1,2,3-triazole derivatives and their synthesis, achieves the effects of high yield of reaction products, mild synthesis reaction conditions, and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] To the 25mL branch tube, add CuBr in sequence 2 (0.09mmol), K 3 PO 4 (0.15mmol), K 2 S 2 o 8 (1.0mmol), S,N-substituted internal olefin 2a (0.3mmol), diazonium salt 3a (0.6mmol), CH 3 CN 3mL, O 2 The reaction was carried out at 25° C. for 5 hours under an atmosphere. After the reaction was over, the solvent was removed under reduced pressure, separated by silica gel column chromatography (the eluent was petroleum ether (60-90° C.) / ethyl acetate, v / v=20:1), and the target product 1a (82 mg , yield 88%). The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 2
[0035]
[0036] The reaction steps and operation are the same as in Example 1, and the difference from Example 1 is that the catalyst is copper chloride. The reaction was stopped, and the target product 1a (72 mg, yield 77%) was obtained after post-treatment.
Embodiment 3
[0038]
[0039] Reaction steps and operation are with embodiment 1, and difference with embodiment 1 is that alkali is K 2 CO 3 . The reaction was stopped, and the target product 1a (65 mg, yield 70%) was obtained after post-processing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com