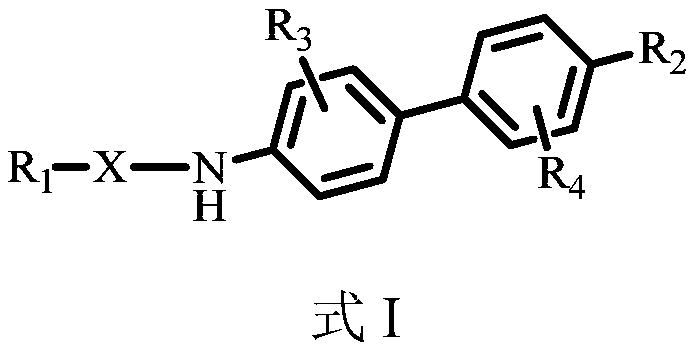
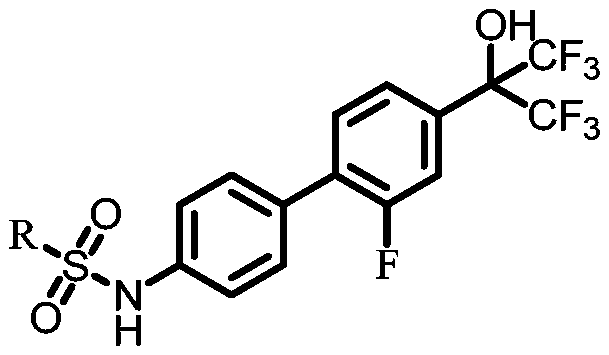
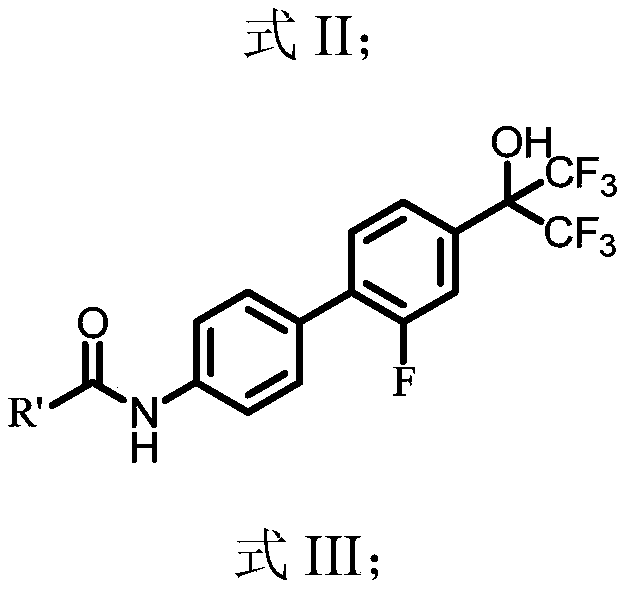
Benzidine compound and application thereof
A compound, benzidine technology, applied in the field of chemical medicine, can solve problems such as no good effect, few structural types of RORγ inhibitors, unpublished druggability data, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] This example provides a benzidine compound: N-(2'-fluoro-4'-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-[1 , 1'-biphenyl]-4-base)-2-(4-(methylsulfonyl)phenyl)acetamide, its preparation method comprises the steps:
[0056] (1) Preparation of 2-(4-amino-3-fluorophenyl)-1,1,1,3,3,3-hexafluoro-2-propanol with the following structure:
[0057]
[0058] Take difluoroaniline (6.0 g, 54 mmol), hexafluoroacetone trihydrate (12.5 g, 56.7 mmol) and p-toluenesulfonic acid (0.85 g, 5.4 mmol) in a pressure vessel. After vacuuming, heat to 90°C with argon protection and react overnight. Cool to room temperature, wash once with saturated sodium bicarbonate, and extract three times with ethyl acetate (3×50 mL). The organic layers were combined, washed once with saturated sodium chloride, dried over anhydrous sodium sulfate, and the organic phase was spin-dried in vacuo. The crude product was separated through a silica gel column (PE:EA=10:1) to obtain 4.45 g of a white solid (90...
Embodiment 2
[0074] This example provides a benzidine compound: 2-(4-(ethylsulfonyl)phenyl)-N-(2'-fluoro-4'-(1,1,1,3,3,3- Hexafluoro-2-hydroxypropane-2-yl)-[1,11,1'-biphenyl]-4-yl)acetamide, its preparation method comprises the steps:
[0075] Steps (1)-(4) are consistent with Example 1, and then step (5) is performed.
[0076] (5) Prepare 2-(4-(ethylsulfonyl)phenyl)-N-(2'-fluoro-4'-(1,1,1,3,3,3-hexafluoro- 2-Hydroxypropan-2-yl)-[1,11,1'-biphenyl]-4-yl)acetamide:
[0077]
[0078] Compound 2-(4-(ethylsulfonyl)phenyl)acetic acid (70.7 mg, 0.31 mmol), diisopropylethylamine (0.5 mL) and HATU (646.4 mg, 1.70 mmol) were dissolved in 20 mL of DCM. The reaction mixture was stirred at room temperature for 5 minutes, then the compound 2-(4'-amino-2-fluoro-[1,1'-biphenyl]-4-yl)-1,1,1,3,3 , 3-hexafluoro-2-propanol (100mg, 0.28mmol), and the resulting mixture was stirred at room temperature for 3 hours. The reaction was monitored by TLC. After the reaction was completed, water was added, extrac...
Embodiment 3
[0082] This example provides a benzidine compound: N-(2'-fluoro-4'-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-[1 , 1'-biphenyl]-4-base)-2-(4-nitrophenyl)acetamide, its preparation method comprises the steps:
[0083] Steps (1)-(4) are consistent with Example 1, and then step (5) is performed.
[0084] (5) Preparation of N-(2'-fluoro-4'-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-[1,1' -biphenyl]-4-yl)-2-(4-nitrophenyl)acetamide:
[0085]
[0086] Compound 2-(4-nitrophenyl)acetic acid (56.2 mg, 0.31 mmol), diisopropylethylamine (0.5 mL) and HATU (646.4 mg, 1.70 mmol) were dissolved in 20 mL of DCM. The reaction mixture was stirred at room temperature for 5 minutes, then the compound 2-(4'-amino-2-fluoro-[1,1'-biphenyl]-4-yl)-1,1,1,3,3 , 3-hexafluoro-2-propanol (100mg, 0.28mmol), and the resulting mixture was stirred at room temperature for 3 hours. The reaction was monitored by TLC. After the reaction was completed, water was added, extracted with ethyl acetate (3×5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com