Method for preparing polylactone
A polylactone and cyclic lactone technology, applied in the field of polylactone preparation, can solve the problems of unfavorable material stability, uncontrollable reaction process, low molecular weight of the product, etc., and achieve narrow molecular weight distribution index and no monomer residue , high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
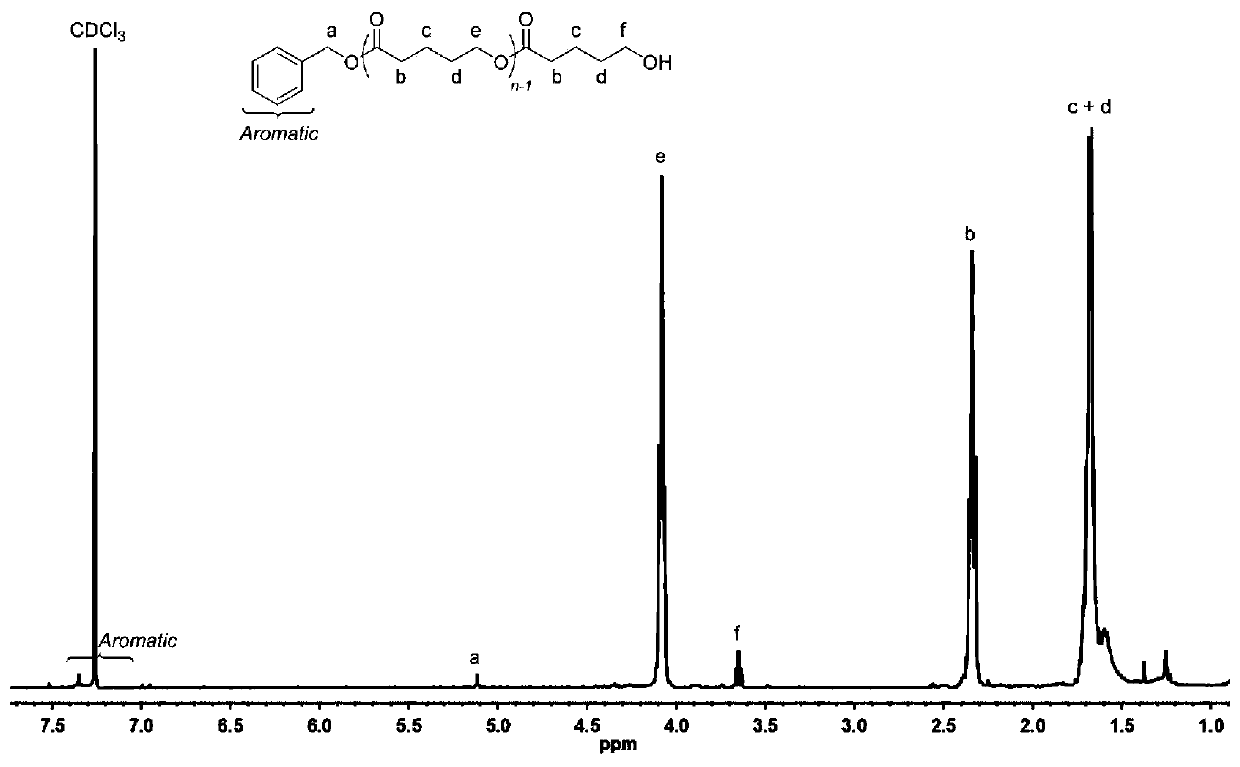
[0055] In a 10mL polymerization tube, add δ-valerolactone (0.27ml, 3mmol), hydantoin derivative (7) (0.016g, 0.1mmol), DBU (14.94ul, 0.1mmol), benzyl alcohol (10.34μL, 0.1mmol), stirred magnetically for 4 hours at 90°C, stopped the reaction, added a small amount of dichloromethane dropwise to the resulting mixture to dissolve, then slowly dropped the resulting solution into cold methanol, a white polymer was precipitated, and was centrifuged and vacuum-dried 0.38 g of the product was obtained, the conversion rate was 95.2%, the number average molecular weight Mn of polyvalerolactone was 3612 g / mol, and the molecular weight distribution PDI was 1.07. The hydrogen spectrum of polyvalerolactone is as follows figure 1 As shown, ( 1 H NMR (CDCl 3 ):δ(ppm),1.67–1.70(m,4H×n,(-CH 2 CH 2 CH 2 O-)n and (-COCH 2 CH 2 CH 2 -)n), 1.95(q, 2H, J=7.2Hz, ArCH 2 CH 2 CH 2 -),2.34(t,2H×n,J=6.9Hz,(-OCOCH 2 CH 2 -)n), 2.68(t, 2H, J=7.7Hz, ArCH 2 CH 2 -),3.65(t,2H,J=6.5Hz,-CH 2 CH ...
Embodiment 2
[0057] In a 10 mL polymerization tube, add D-lactide (0.072 g, 0.5 mmol), hydantoin derivative (3) (0.002 g, 0.01 mmol), 2-tert-butyl-1,1,3,3- Tetramethylguanidine (20.4ul, 0.1mmol), pentaerythritol (9.7μL, 0.1mmol), magnetically stirred at 200°C for 24 hours, stopped the reaction, added a small amount of dichloromethane dropwise to the resulting mixture to dissolve, and then dissolved the resulting solution Slowly drop into cold methanol, a white polymer precipitated, centrifuged and vacuum dried to obtain 0.034g of the product, the conversion rate was 67.9%, the number average molecular weight Mn of poly-D-lactide was 1310g / mol, and the molecular weight distribution PDI was 1.19.
Embodiment 3
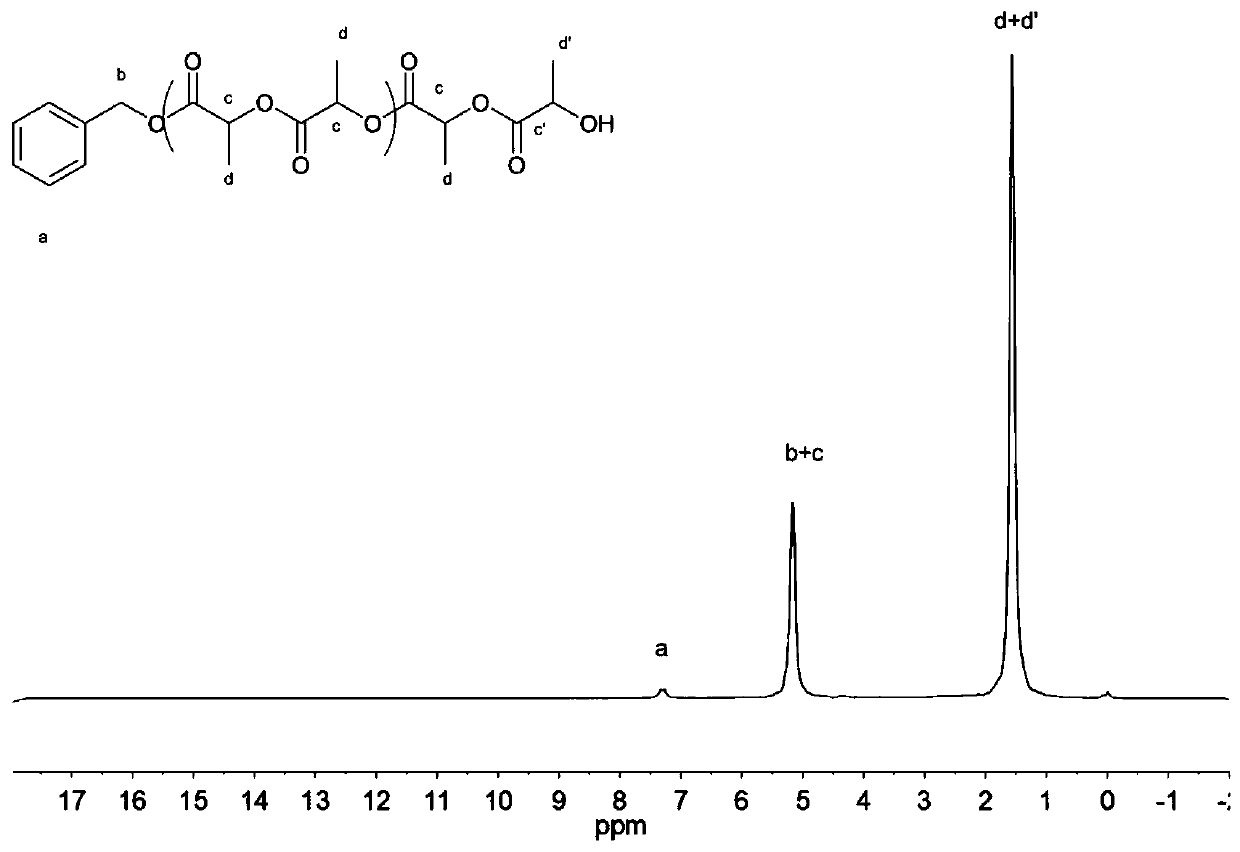
[0059] In a 10mL polymerization tube, add L-lactide (7.2g, 50mmol), hydantoin derivative (4) (0.017g, 0.1mmol), tetramethylguanidine (12.55ul, 0.1mmol), benzyl alcohol ( 10.0 μL, 0.1 mmol), stirred magnetically at 130°C for 8 hours, stopped the reaction, added a small amount of dichloromethane dropwise to the resulting mixture to dissolve, then slowly dropped the resulting solution into cold methanol, a white polymer was precipitated, and centrifuged , Vacuum drying to obtain 5.9 g of the product, the conversion rate is 98.4%, the number average molecular weight Mn of poly-L-lactide is 37100 g / mol, and the molecular weight distribution PDI is 1.20. The hydrogen spectrum of polylactide is as image 3 As shown, ( 1 H NMR (400MHz, CDCl 3 ),δ(ppm),1.57-1.59(m,3H×n,(–CH 3 )n),4.34(m,1H,–CH(CH 3 )OH),5.13–5.21(q,1H×(n-1),–CH(CH 3 )O–; 2H, ArCH 2 O–), 7.33–7.34 (m, 5H, aryl).); Its size exclusion chromatography analysis spectrum is as Figure 4 As shown, it can be seen from th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com