Alkaline oxygen reduction reaction catalyst, preparation method and application thereof
A catalyst and reaction technology, applied in nanotechnology, structural parts, electrical components, etc. for materials and surface science, can solve the problem of insufficient manganese cobalt spinel activity, insufficient catalytic oxygen reduction activity, manganese cobalt spinel catalyst Insufficient catalytic oxygen reduction activity and other problems, to achieve the effect of high oxygen reduction reaction catalytic activity and improve controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] (1) Ultrasonically disperse 60mg of carbon carrier (solid carbon spheres with a particle size of 30nm) in 15mL of cobalt acetate aqueous solution with a concentration of 17.04mmol / L, then add 0.5mL of ammonia water with a concentration of 14mol / L dropwise, and stir in an air atmosphere 10min, obtain solution A;
[0078] (2) adding dropwise 15mL concentration in the solution A that step (1) obtains is 17.04mmol / L manganese acetate aqueous solution, obtains solution B;
[0079] (3) Aging solution B at 60°C for 120min, then adding it into a 35mL closed reaction kettle, and performing solvothermal reaction at 150°C for 3h;
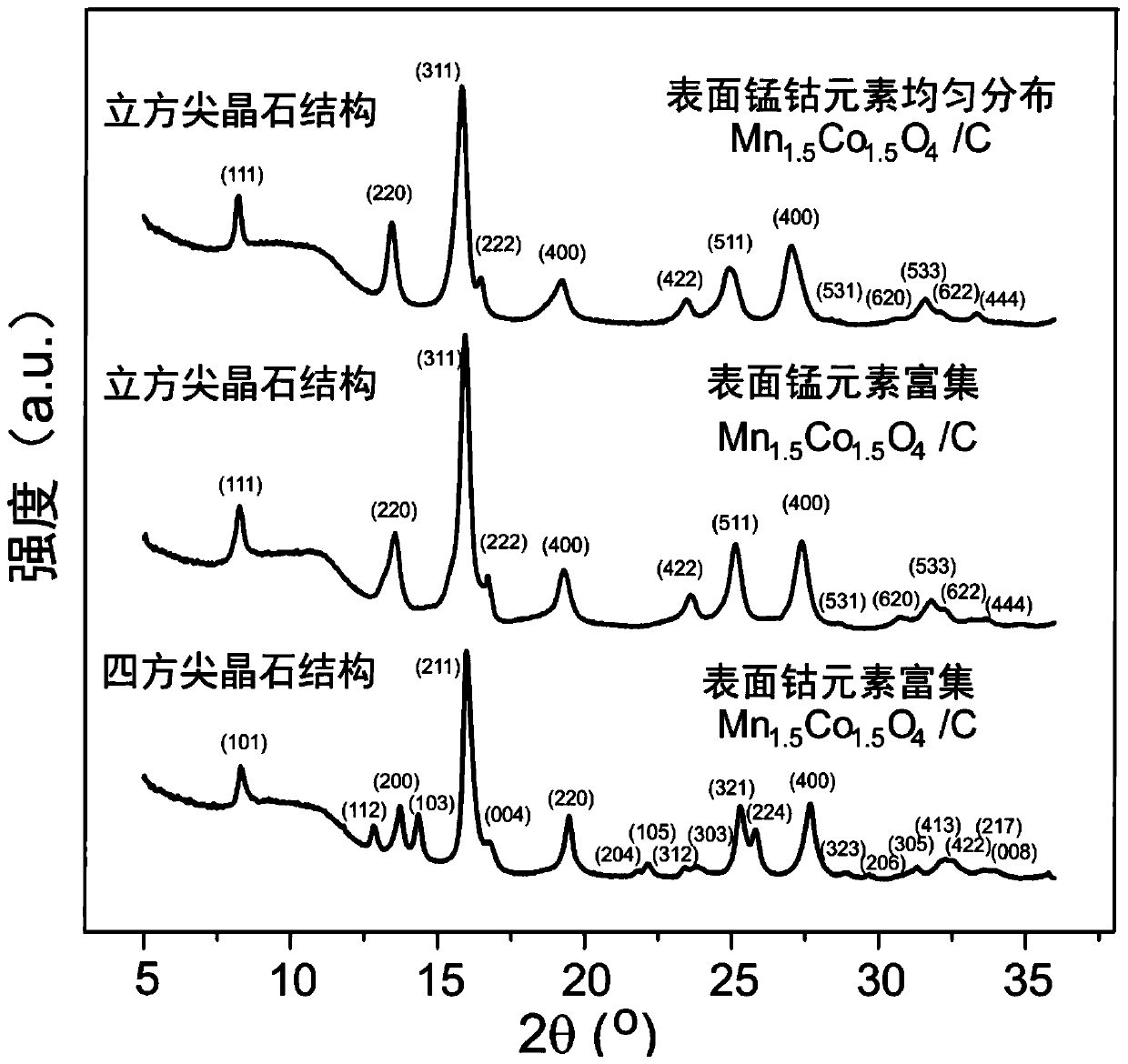
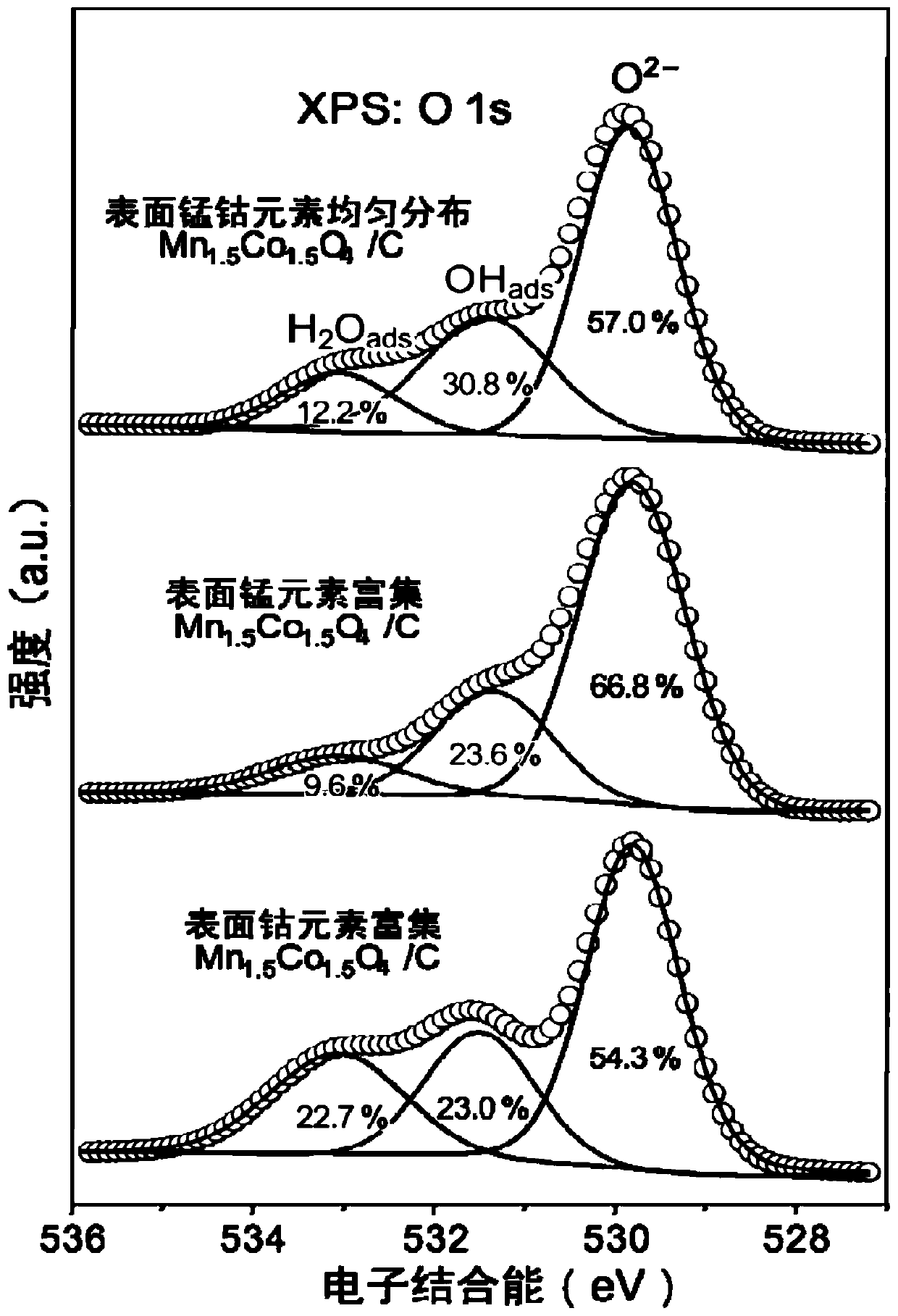
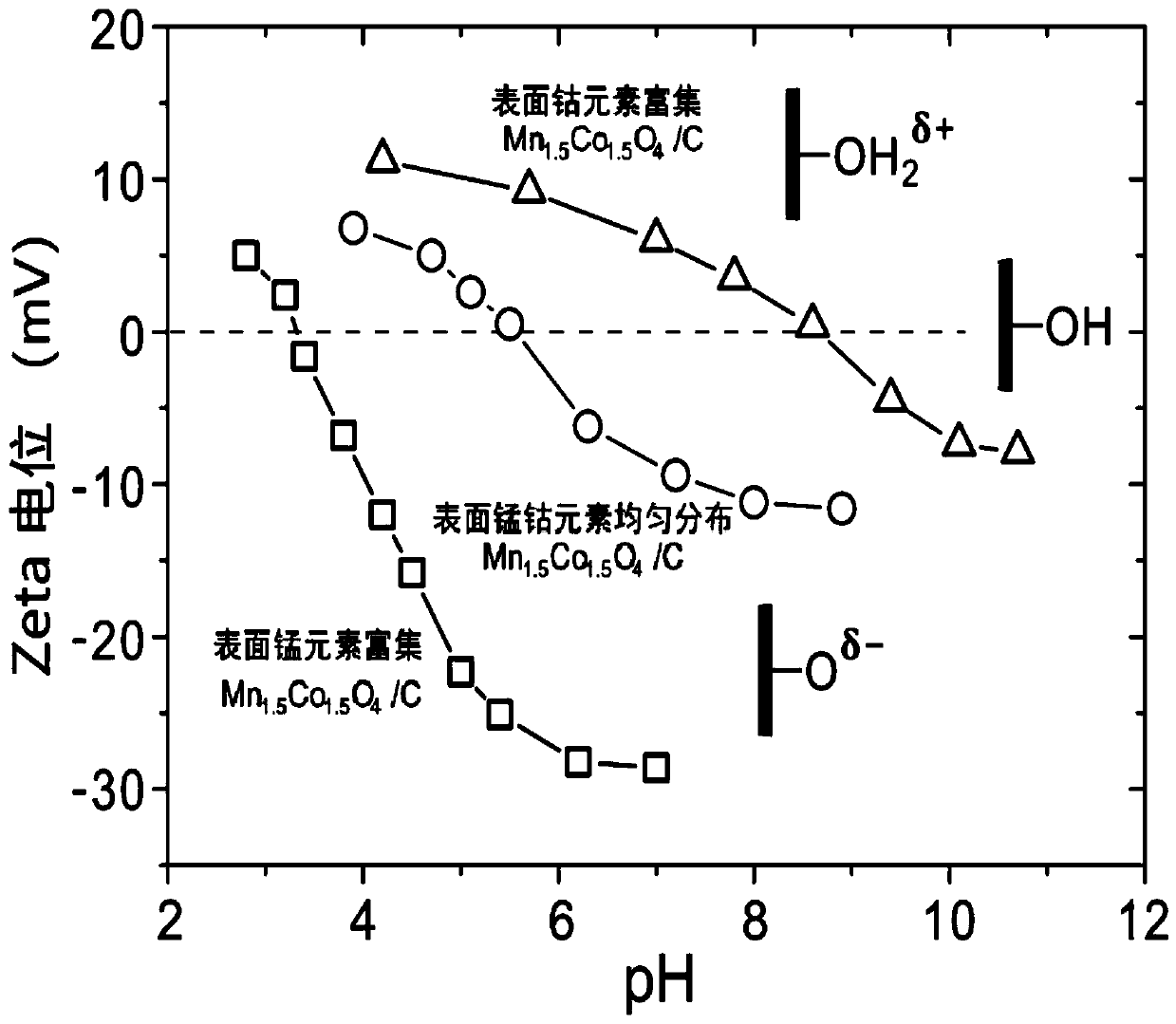
[0080] (4) The product of step (3) is naturally cooled to room temperature, centrifuged, washed and dried to obtain an alkaline oxygen reduction reaction catalyst, which is recorded as the surface manganese and cobalt elements evenly distributed Mn 1.5 co 1.5 o 4 / C.
[0081] In the alkaline oxygen reduction reaction catalyst prepared in this exampl...
Embodiment 2
[0083] In this example, the aging temperature of step (3) in Example 1 is replaced by 40°C, and the other conditions are exactly the same as those in Example 1, and the basic oxygen reduction reaction catalyst is prepared, which is recorded as the surface manganese element enriched Mn 1.5 co 1.5 o 4 / C.
[0084] In the alkaline oxygen reduction reaction catalyst prepared in this example, the mass ratio of carbon support to spinel is 0.67:1, and the spinel is Mn 1.5 co 1.5 o 4 The nanoparticle has an average particle diameter of 20nm; the surface of the spinel nanoparticle is rich in manganese, and the molar ratio of manganese to cobalt on the surface is 1.35:1.
Embodiment 3
[0086] In this example, the aging temperature of step (3) in Example 1 is replaced by 80°C, and the other conditions are exactly the same as those in Example 1, and a basic oxygen reduction reaction catalyst is prepared, which is recorded as surface cobalt enriched with Mn 1.5 co 1.5 o 4 / C.
[0087] In the alkaline oxygen reduction reaction catalyst prepared in this example, the mass ratio of carbon support to spinel is 0.67:1, and the spinel is Mn 1.5 co 1.5 o 4 The nanoparticles have an average particle size of 20nm; the surface of the spinel nanoparticles is enriched in cobalt, and the molar ratio of manganese to cobalt on the surface is 0.5:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com