Method for preparing AFP (alpha fetoprotein) monoclonal antibody by in-vitro culture and application of AFP monoclonal antibody
A monoclonal antibody and in vitro culture technology, applied in instruments, analytical materials, measuring devices, etc., can solve the problems of easy pollution, troublesome manual operation, etc., and achieve the effects of long-term growth, simplified operation, and optimized process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of AFP monoclonal antibody by in vitro culture using hollow fiber system
[0025] The first step is to recover hybridoma cells and prepare seed cells
[0026] (1) Before the experiment, the ultra-clean workbench needs to be disinfected for 30 minutes, the wind speed is set to level 3, ventilated for 15 minutes, the measured wind speed is greater than 0.3m / s, and the equipment usage records and wind speed measurement records are filled in. Wipe and disinfect the surface of the ultra-clean workbench, required consumables, the surface of reagent bottles and hands with 75% alcohol.
[0027] (2) Preheat the required DMEM medium and serum in a 37°C water bath, and then configure DMEM medium containing 15% newborn bovine serum (collectively referred to as complete medium) in the ultra-clean workbench.
[0028] (3) After the hybridoma cells were taken out from the liquid nitrogen, the cell suspension was sucked out, and slowly added to a centrifuge tube p...
Embodiment 2
[0048] Example 2 Purification of AFP monoclonal antibody (purify the in vitro antibody sample to be purified harvested in Example 1)
[0049] 1. Harvested in vitro antibody sample processing
[0050] (1) First detect the concentration of the in vitro antibody sample to be purified: take 2 microliters of the sample and use the UV spectrophotometer IgG mode to detect its content, and the blank control is 0.02M PBS with pH 7.4.
[0051] (2) Pass the collected in vitro antibody samples through a 0.45 μm filter membrane.
[0052] 2. Purification process
[0053] (1) Initial pump washing pipeline: Open the PrimeView software, put all the inlets of AKTAprime plus into filtered and purified water, operate the machine, enter Templates / Application Template / System Wash Method in turn, select B, A1, A3, A4, A8, press the "OK" button, the system will automatically flush.
[0054] (2) Place the injection ports of all pipelines into corresponding reagent bottles;
[0055] (3) Pump and wa...
Embodiment 3
[0061] Example 3 Performance evaluation of the AFP monoclonal antibody prepared by the present invention
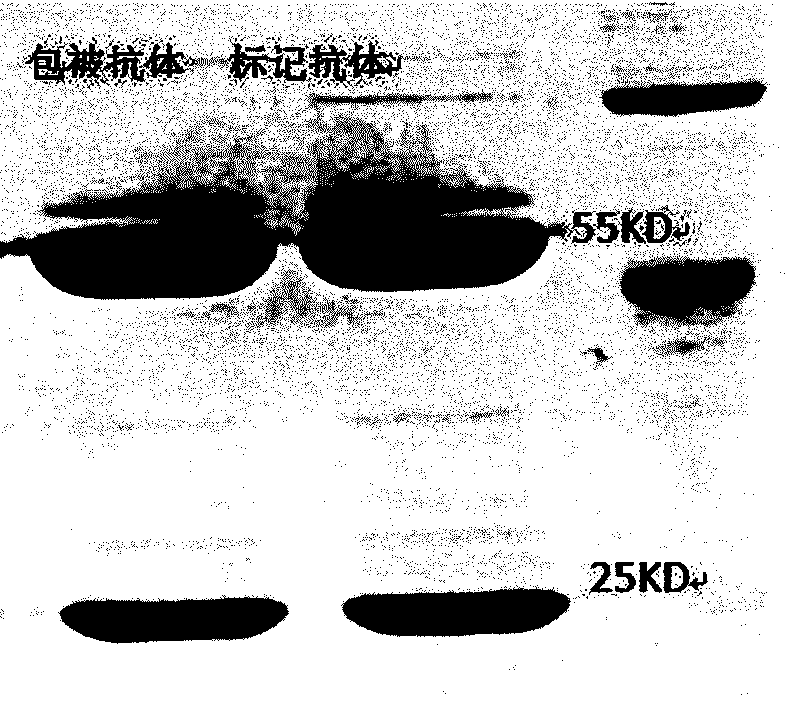
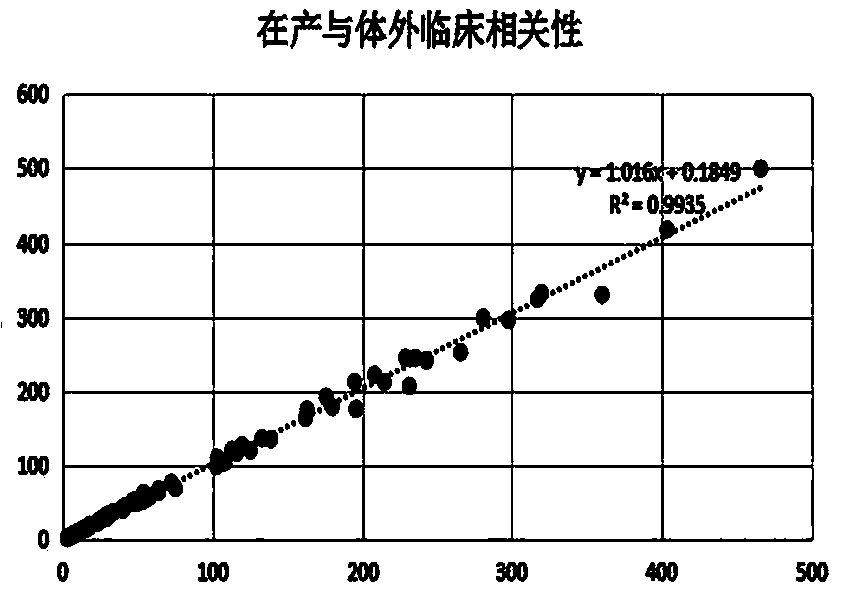
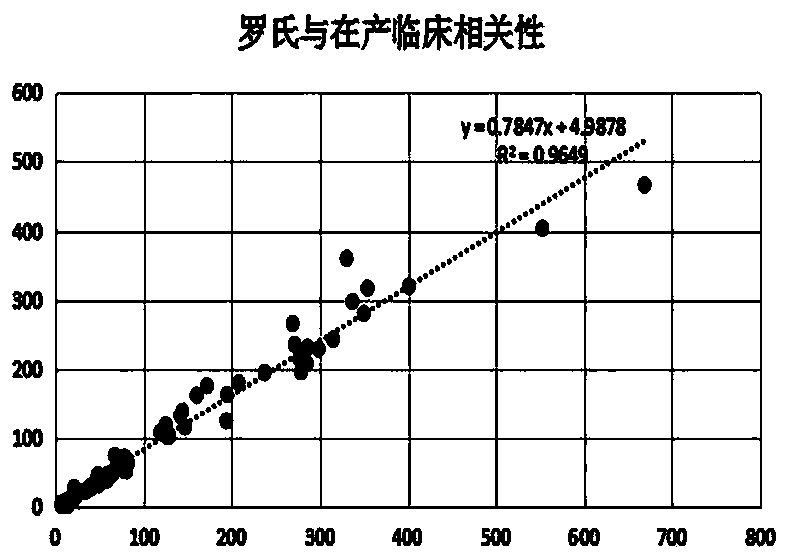
[0062] The chemiluminescent AFP detection kit is used for detection. The principle of the kit is the double-antibody sandwich method to detect antigens. The monoclonal antibodies (hereinafter referred to as in vitro antibodies) prepared by the present invention are used as coating antibodies and labeled antibodies respectively, and compared with the kits in production. .
[0063] 1. Sensitivity assessment
[0064] Measure the zero point (S0) of 10 standard products, calculate the average value of luminescence and its standard deviation, and then bring the value obtained by adding twice the standard deviation (SD) of the average value of luminescence (`X) into the standard curve to obtain the corresponding concentration value. The sensitivity test is shown in Table 1 below.
[0065] Table 1 Sensitivity comparison of in-produced antibody and in vitro antibody preparation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com