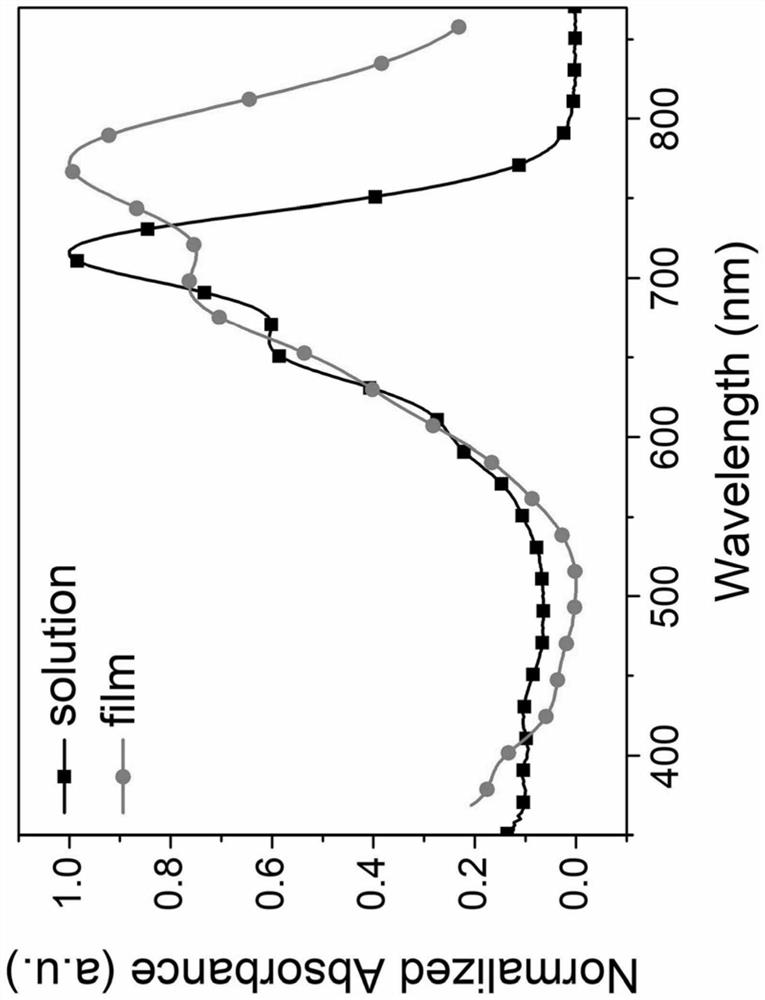
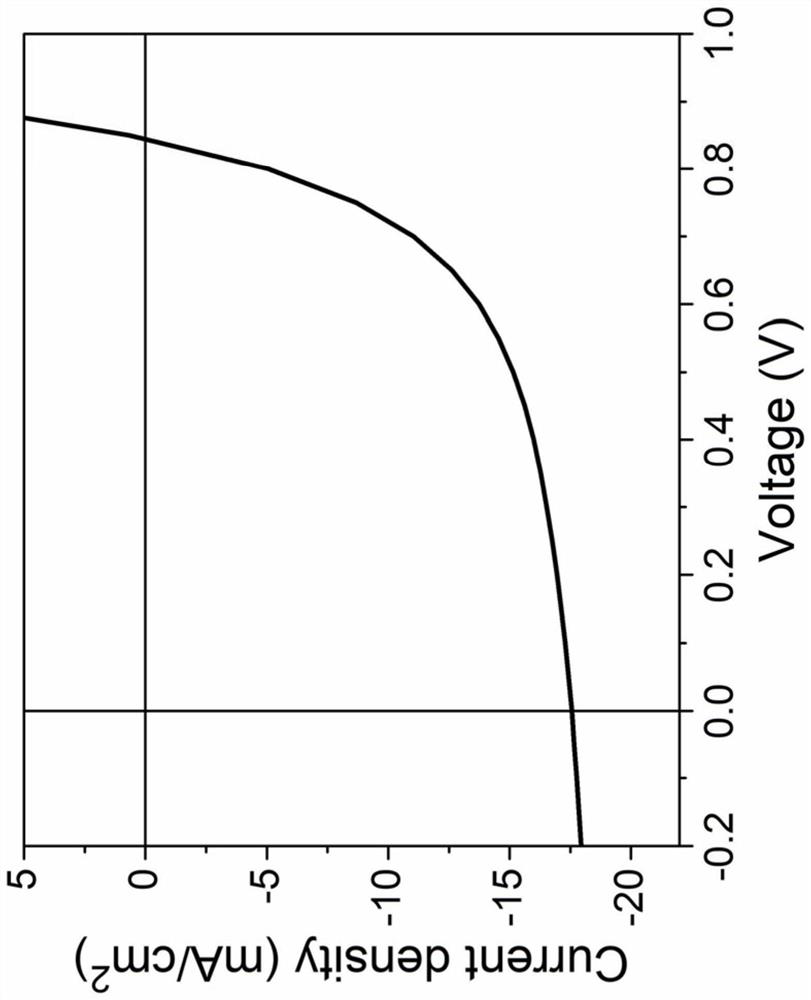
A kind of asymmetric naphthalene nuclear small molecule acceptor material and its preparation method and application
A naphthalene-nuclear small molecule and acceptor material technology, applied in the field of organic solar cells, can solve the problems of narrow absorption spectrum, limited photocurrent and energy conversion efficiency, and achieve the goal of improving utilization rate, improving energy conversion efficiency, and broadening the absorption range Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The preparation method of the asymmetric naphthalene nuclear small molecule acceptor material CPTT-IC, the specific steps are as follows:
[0075]
[0076]
[0077] (1) Synthesis of Compound 1: Naphthalene-1,4-bisboronic acid pinacol ester (1.32g, 3.47mmol), 2-bromothieno[3,2-b]thiophene-3-carboxylic acid ethyl ester ( 2.53g, 8.68mmol), 45mL toluene, 20mL ethanol, 20mL K 2 CO 3 The solution (2mol / L) was added to a 250mL reaction flask, and 180mg of tetrakis(triphenylphosphine)palladium was added under the protection of argon, and refluxed for 24h. Pour into water after cooling, extract with dichloromethane, dry over anhydrous magnesium sulfate, spin off the solvent, and purify the crude product by column chromatography, using petroleum ether / dichloromethane (2:1) as eluent, to obtain 1.66g gray solid , and the yield was 88.1%. 1 H NMR (400MHz, CDCl 3 )δ: 7.80(s,2H),7.61(s,2H),7.54(d,J=5.1Hz,2H),7.45-7.39(m,2H),7.34(d,J=5.0Hz,2H), 4.06(br,4H), 0.85(br,6H).
...
Embodiment 2
[0086] A kind of preparation method of asymmetric naphthalene nuclear small molecule acceptor material CPTT-2FIC, the main technical scheme of this embodiment 2 is basically the same as the (1) to (4) steps of embodiment 1, in this embodiment 2 no For the features explained, the explanations in Embodiment 1 are adopted, and details are not repeated here. The difference between this embodiment and embodiment 1 is:
[0087]
[0088]Add compound 4 (110mg, 0.10mmmol), 5,6-difluoro-3-(dicyanomethylene) indoketone (135mg, 0.59mmol) and 30mL chloroform in sequence to a 100mL reaction flask, and add under nitrogen protection 0.5mL pyridine, heated to reflux and reacted overnight, after cooling, precipitated with methanol, the crude product was purified by column chromatography, petroleum ether / dichloromethane (1:1) was the eluent, and 85mg of blue-black solid was obtained. The yield was 56.1%. 1 H NMR (500MHz, CDCl 3 )δ: 8.84(s,1H), 8.72(s,1H), 8.53-8.48(m,2H), 8.15(s,1H), 8.12(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com