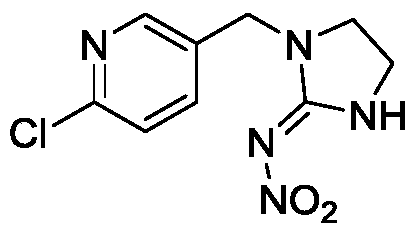
Method for analyzing content of imidacloprid synthetic intermediate through liquid chromatography-mass spectrometry
A technology of imidacloprid and combined use, applied in the field of analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
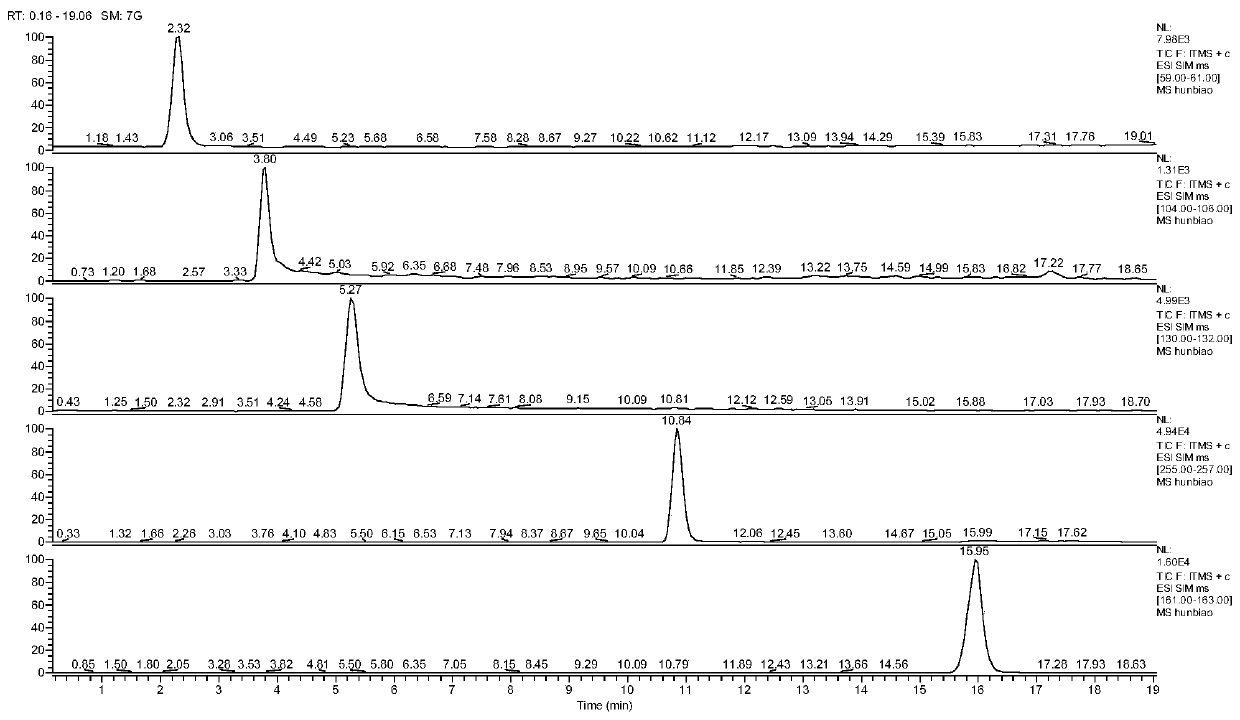
[0021] (1) Experimental instruments and reagents
[0022] (1) Experimental equipment: The instrument used is a Thermo Fisher ultra-high performance liquid chromatography-ion trap mass spectrometer, and the chromatographic column is an Agilent Eclipse PluC18 liquid chromatographic column (4.6×100mm, 3.5μm); a syringe-type microporous organic filter membrane (0.22μm);
[0023] (2) Experimental reagents: methanol, Watsons distilled water, ammonium acetate, perfluorovaleric acid, guanidine nitrate, nitroguanidine, imidazolidine, 2-chloro-5-chloromethylpyridine and imidacloprid standard substances;
[0024] (2) Chromatography and mass spectrometry conditions
[0025] (3) Liquid phase conditions: mobile phase A is methanol; mobile phase B is 0.02mol / L ammonium acetate aqueous solution (containing 0.04 / % perfluorovaleric acid); flow rate is 0.3mL / min, gradient elution program: 0-5min : A: 15%, the remaining components are B; 5.1-15min: A: 75%, the remaining components are B; 20.1-2...
Embodiment 2
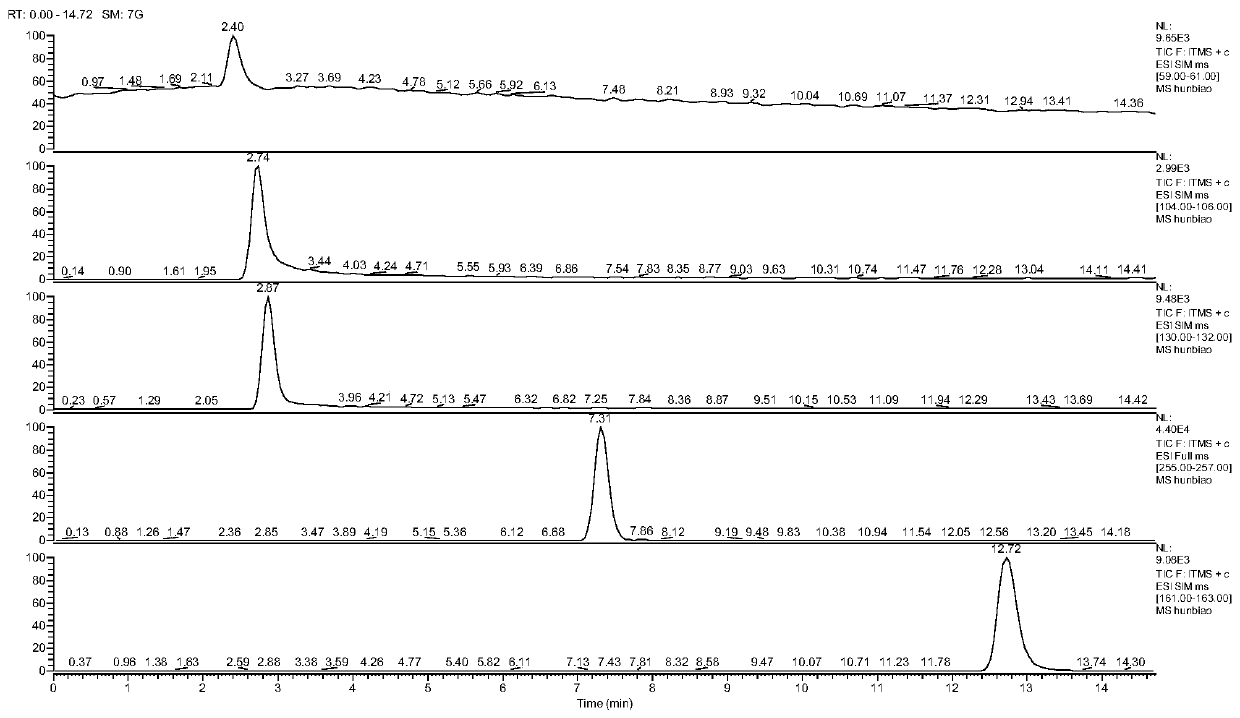
[0034] (1) Experimental instruments and reagents
[0035](1) Experimental equipment: The instrument used is a Thermo Fisher ultra-high performance liquid chromatography-ion trap mass spectrometer, and the chromatographic column is an Agilent Eclipse PluC18 liquid chromatographic column (4.6×100mm, 3.5μm); a syringe-type microporous organic filter membrane (0.22μm);
[0036] (2) Experimental reagents: methanol, Watsons distilled water, ammonium acetate, perfluoroheptanoic acid, guanidine nitrate, nitroguanidine, imidazolidine, 2-chloro-5-chloromethylpyridine and imidacloprid standard substances;
[0037] (2) Chromatography and mass spectrometry conditions
[0038] (3) Liquid phase conditions: mobile phase A is methanol; mobile phase B is 0.01mol / L ammonium acetate aqueous solution (containing 0.06 / % perfluoroheptanoic acid); flow rate is 0.5mL / min, gradient elution program: 0-5min : A: 10%, the remaining components are B; 5.1-15min: A: 80%, the remaining components are B; 20....
Embodiment 3
[0048] (1) Experimental instruments and reagents
[0049] (1) Experimental equipment: The instrument used is a Thermo Fisher ultra-high performance liquid chromatography-ion trap mass spectrometer, and the chromatographic column is an Agilent Eclipse PluC18 liquid chromatographic column (4.6×100mm, 3.5μm); a syringe-type microporous organic filter membrane (0.22μm);
[0050] (2) Experimental reagents: methanol, Watsons distilled water, ammonium acetate, perfluorooctanoic acid, guanidine nitrate, nitroguanidine, imidazolidine, 2-chloro-5-chloromethylpyridine and imidacloprid standard
[0051] (2) Chromatography and mass spectrometry conditions
[0052] (3) Liquid phase conditions: mobile phase A is methanol; mobile phase B is 0.015mol / L ammonium acetate aqueous solution (containing 0.1 / % perfluorooctanoic acid); flow rate is 0.3mL / min, gradient elution program: 0-5min: A: 25%, the remaining components are B; 5.1-15min: A: 70%, the remaining components are B; 20.1-25min: A: 25...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com