Preparation method of 4-cyclopropyl-1-naphthylamine
A technology of cyclopropyl and naphthylamine, which is applied in the field of preparation of Lesinurad intermediates, can solve problems such as difficult application, and achieve the effect of reducing production cost and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
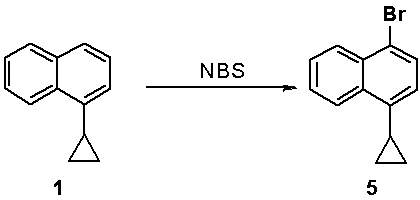
[0034] The synthesis of embodiment 1,4-cyclopropyl-1-bromonaphthalene
[0035]
[0036] 1-Cyclopropylnaphthalene (5.0 g, 30 mmol) was dissolved in 15 mL of acetic acid, liquid bromine (5.68 g, 36 mmol) was added dropwise, and the mixture was reacted at 10-20 °C for 1-2 hours. After the reaction was completed, the reaction solution was quenched with water, extracted with dichloromethane, washed with alkali, washed with sodium sulfite solution, washed with water, concentrated to remove dichloromethane, and purified by silica gel column to obtain 6.24 g of 4-cyclopropyl-1-bromonaphthalene. The yield is 85%.
[0037] Product NMR data: 1 H NMR (400 MHz, DMSO) δ 8.51 – 8.42 (m, 1H), 8.23 –8.13 (m, 1H), 7.76 (d, J = 7.7 Hz, 1H), 7.73 – 7.66 (m, 2H), 7.17 (d, J = 7.7Hz, 1H), 2.38 (m, 1H), 1.11 – 1.01 (m, 2H), 0.78 – 0.68 (m, 2H).
Embodiment 2
[0038] The synthesis of embodiment 2,4-cyclopropyl-1-bromonaphthalene
[0039]
[0040] 1-Cyclopropylnaphthalene (5.0 g, 30 mmol) was dissolved in 50 mL of acetonitrile, NBS (7.1 g, 40 mmol) was added, and the mixture was reacted at 50 °C for 24 hours. After the reaction was completed, the reaction liquid was cooled to room temperature, concentrated to remove acetonitrile, added 50 mL of n-heptane, stirred and filtered. The filtrate was concentrated and then purified by a silica gel column to obtain 6.7 g of 4-cyclopropyl-1-bromonaphthalene with a yield of 90%.
Embodiment 3
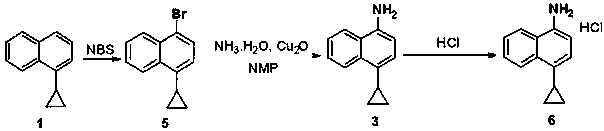
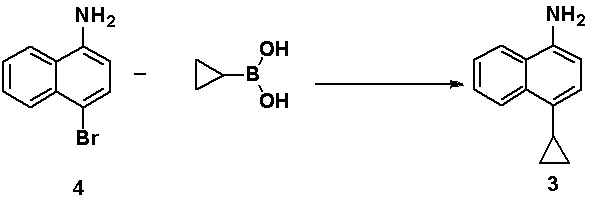
[0041] The synthesis of embodiment 3,4-cyclopropyl-1-naphthylamine
[0042]
[0043] 4-Cyclopropyl-1-bromonaphthalene (6.0 g, 24 mmol) was dissolved in 12 mL of N, N-dimethylformamide (DMF), added cuprous oxide (0.52 g, 3.6 mmol), ammonia water 12 mL (Ammonia mass concentration 25%), airtight autoclave and press into ammonia gas 20-30min. The mixed system was reacted at 100 °C for 28 hours. After the reaction was completed, the reaction liquid was cooled to room temperature, 36 ml of water was added, extracted twice with 70 mL of methyl tert-butyl ether, concentrated, and purified by a silica gel column to obtain 3.56 g of 4-cyclopropyl-1-naphthylamine , the yield is 80%.
[0044] Product NMR data: 1 H NMR (400 MHz, DMSO) δ 8.25 (d, J = 7.9 Hz, 1H), 8.07(d, J = 8.0 Hz, 1H), 7.48 (m, 1H), 7.39 (m, 1H), 6.99 (m, 1H), 6.59 (d, J =7.7 Hz, 1H), 5.50 (s, 2H), 2.14 (m, 1H), 1.00 – 0.83 (m, 2H), 0.61 – 0.47 (m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com