Drug-loaded balloon and preparation method thereof
A balloon and drug-loading technology, which is applied in medical science, balloon catheters, surgery, etc., can solve the problems of insufficient gene quantity, difficulty in exerting curative effect, easy loss, etc., and achieve the effect of avoiding excessive loss of genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
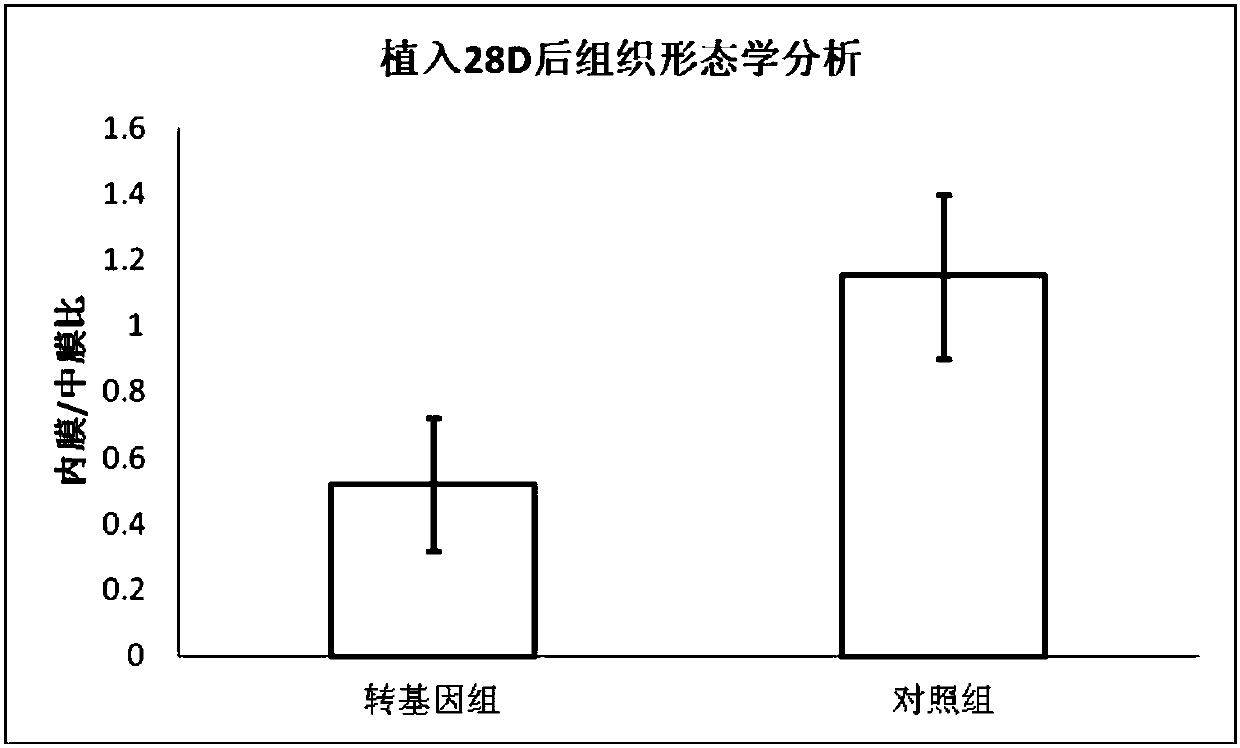
Image
Examples
preparation example Construction
[0042] The preparation method of the drug-loaded balloon of one embodiment, comprises the following steps:
[0043] S110: A balloon is provided, and a drug-loaded layer is prepared on the outer surface of the balloon, and the drug-loaded layer contains a gene.
[0044] The balloon can be a round balloon, a cylindrical balloon or a balloon of other shapes.
[0045] The drug-loaded layer contains genes and gene carriers. The types and proportions of the gene and the matrix carrier are the same as above, and will not be repeated here.
[0046] Preferably, the drug-loaded layer also contains a stabilizer, and the types and proportions of the stabilizer are the same as above, and will not be repeated here.
[0047] Mix the gene, the gene carrier and the stabilizer evenly to prepare the first coating solution, apply the first coating solution on the outer surface of the balloon by spraying, dipping, dripping and other coating methods, and dry it on the balloon The outer surface f...
Embodiment 1
[0066] (1) Place the balloon in the plasma transmitter for surface treatment. Treatment conditions: power 500W, time 30min, atmosphere: mixed atmosphere of argon and oxygen, the flow rates of argon and oxygen are both 75sccm;
[0067] (2) Use RNase-free H 2 The has-miR-1298-5p mimic gene (purchased from Guangzhou Ruibo Biotechnology Co., Ltd., specification 5nmol) was dissolved in O to prepare a 200nM gene solution. Poloxamer P188 was treated with sterile ddH 2 O was configured as a 5mg / mL gene carrier solution. The above gene solution and gene carrier solution were mixed at a volume ratio of 8:2 to prepare the first coating solution. Each 100μL of the first coating solution contains 16×10 -3 nmol of has-miR-1298-5p mimic gene;
[0068] (3) Take 200 μL of the first coating solution with a syringe, slowly drop-coat it on the surface of the balloon, and repeatedly drop-coat it after drying until all the first coating solution is used up, and form a drug-loaded layer on the ...
Embodiment 2
[0072] (1) Place the balloon in the plasma transmitter for processing. Treatment conditions: power 700W, time 10min, atmosphere: mixed atmosphere of argon and oxygen, the flow rates of argon and oxygen are both 75sccm;
[0073] (2) with sterilized ddH 2 O dissolved the miR-21antagomir gene (purchased from Guangzhou Ruibo Biotechnology Co., Ltd., specification 0.5nmol), and prepared a 10nM gene solution. Gum Arabic and Tween were mixed at a ratio of 8:2 (mass ratio), and a gene carrier solution with a total concentration of 1 mg / mL was prepared with ethanol with a volume fraction of 20%. The above gene solution and gene carrier solution were mixed at a volume ratio of 5:5 to prepare the first coating solution. Each 100μL of the first coating solution contains 0.5×10 -3 nmol of miR-21antagomir gene;
[0074] (3) Use a syringe to take 500 μL of the first coating solution, slowly drop-coat it on the surface of the balloon, and repeatedly drop-coat it after drying until the fir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com