A kind of synthetic method of epoxy resin of many epoxy groups
A multi-epoxy epoxy resin and synthetic method technology, applied in the direction of organic chemistry, etc., to achieve the effects of mild synthesis reaction conditions, high yield, and good hydrolysis resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A kind of synthetic method of epoxy resin of many epoxy groups, comprises the following steps;
[0027] S1: Put allyl glycidyl ether and 2,3-dimercapto-1-propanol in a molar ratio of 2:1 into the reactor, and add allyl glycidyl ether and 2,3-dimercapto-1-propanol 3% of the total mass of 1-propanol, benzoin dimethyl ether and allyl glycidyl ether and 1,4-dioxane of 1 times the total mass of 2,3-dimercapto-1-propanol, and in a UV lamp Under irradiation (light intensity is 10000μW / cm 2 ), reacted in an ice-water bath for 2.0 hours to obtain the reaction solution of the reaction intermediate.
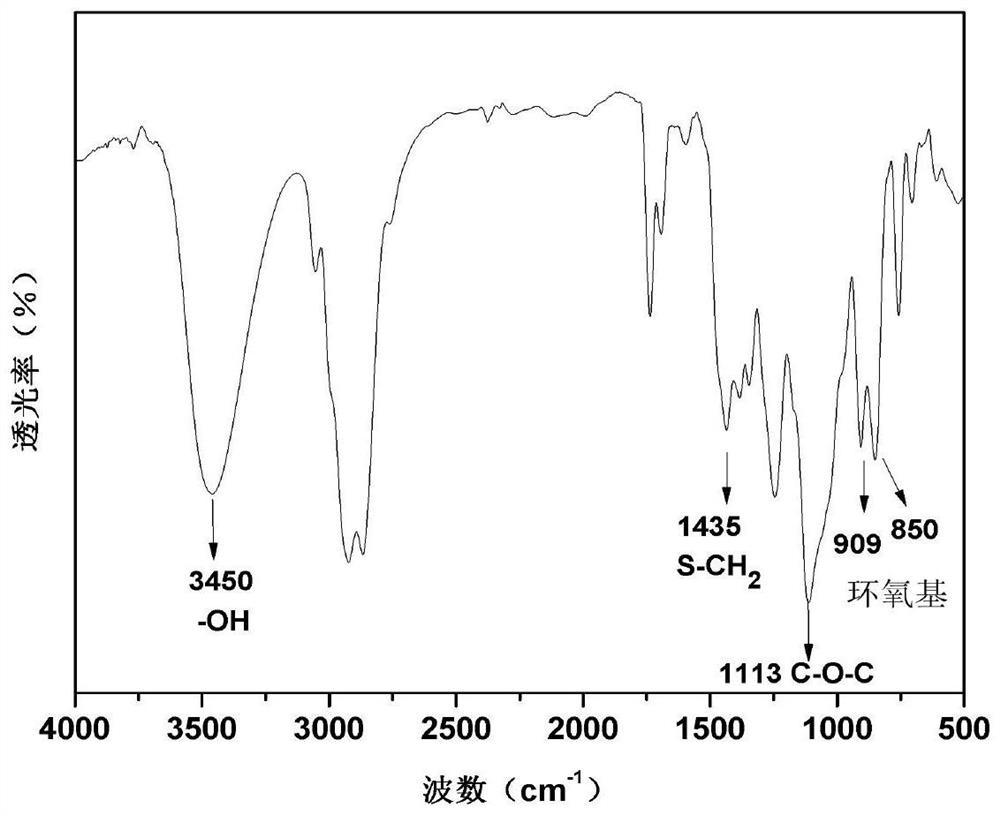
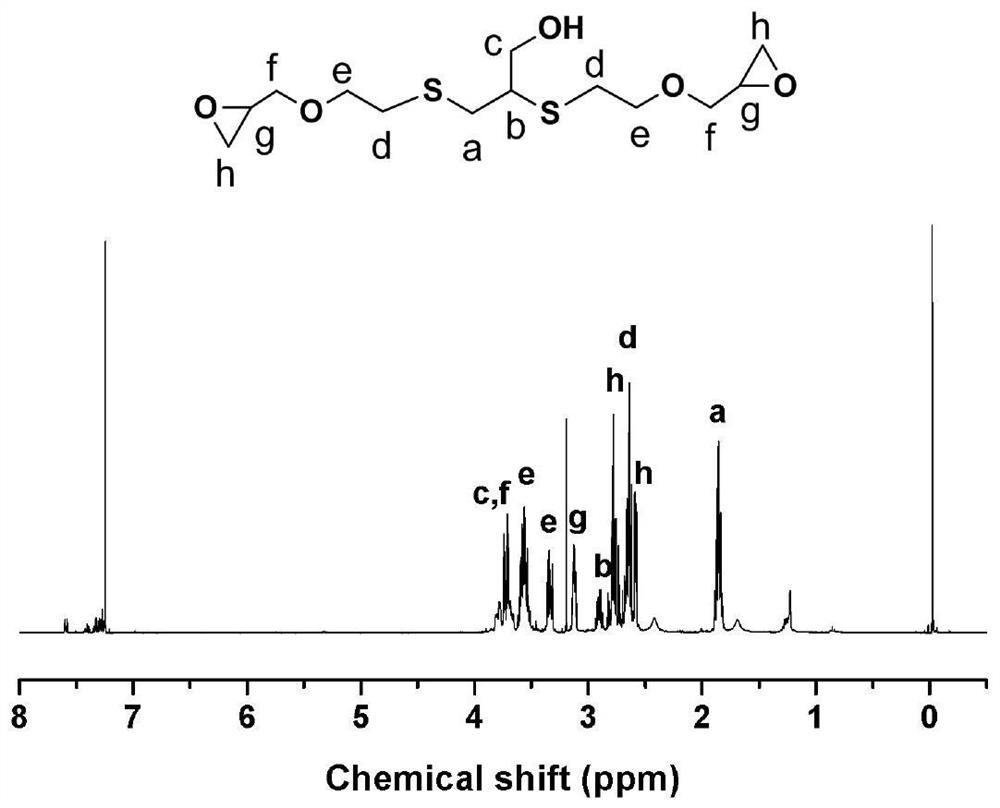
[0028] The reaction solution can be concentrated on a rotary evaporator to obtain an intermediate of α-epoxy-ω-hydroxyl. Its chemical structure was characterized by infrared spectroscopy, and the results are as follows: figure 1 shown. At wavenumbers 850 and 909cm -1 The absorption peak at is the characteristic absorption peak of the glycidyl ether type epoxy group, and the wave...
Embodiment 2
[0033] A kind of synthetic method of epoxy resin of many epoxy groups, comprises the following steps;
[0034] S1: Put allyl glycidyl ether and 2,3-dimercapto-1-propanol in a molar ratio of 2.1:1 into the reactor, and add allyl glycidyl ether and 2,3-dimercapto - 3% of the total mass of 1-propanol 2-hydroxymethylphenyl propane-1-one and allyl glycidyl ether and 4 times the total mass of tetrahydrofuran of 2,3-dimercapto-1-propanol, and in UV Under lamp irradiation (light intensity is 10000μW / cm 2 ), reacted in the ice-water bath for 12 hours, and obtained the α-epoxy group-ω-hydroxyl intermediate solution after the reaction;
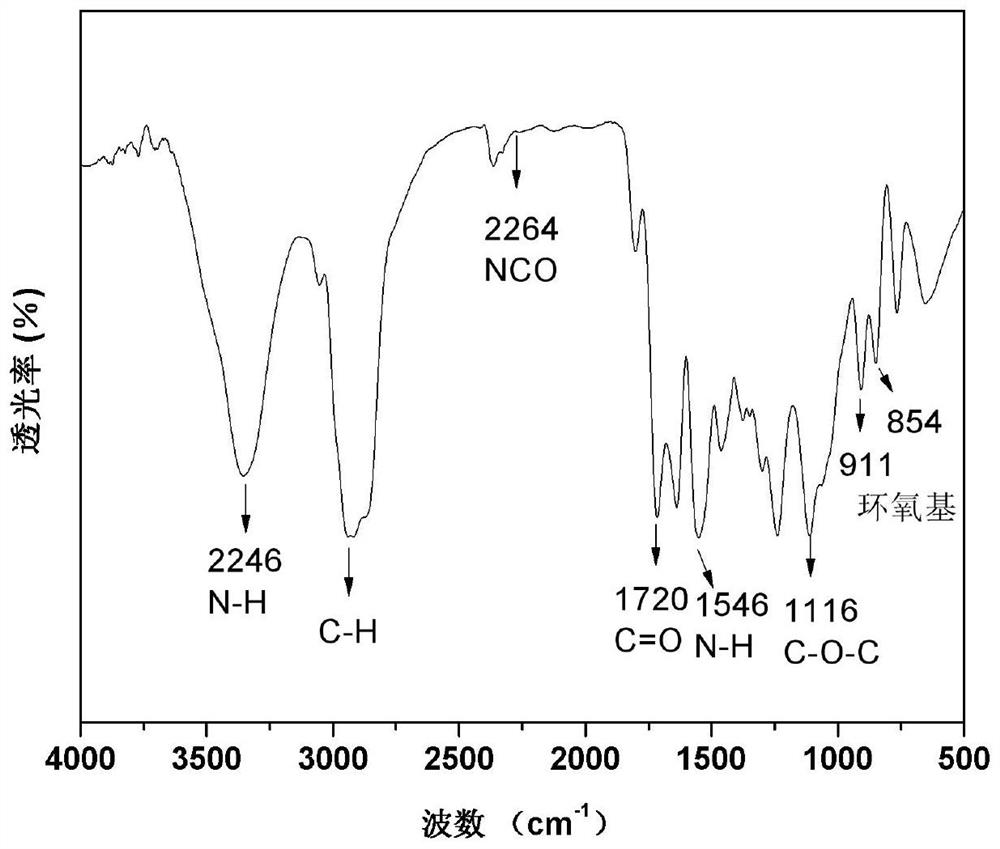
[0035] S2: Add toluene diisocyanate to the above intermediate solution according to the molar ratio of toluene diisocyanate to hydroxyl group 1.1:1, and react at 65°C for 0.5 hours. After the reaction is finished, the solvent is removed from the reaction product under the action of a rotary evaporator; the product is dried in a vacuum oven to obtain a ...
Embodiment 3
[0039] A kind of synthetic method of epoxy resin of many epoxy groups, comprises the following steps;
[0040] S1: Put allyl glycidyl ether and 2,3-dimercapto-1-propanol in a molar ratio of 2.5:1 into the reactor, and add allyl glycidyl ether and 2,3-dimercapto 2-methyl-1-[4-methylthiophenyl]-2-morpholino-1-propanone and allyl glycidyl ether and 2,3-dimercapto with 3% of the total mass of 1-propanol -N,N-dimethylformamide with 3 times the total mass of 1-propanol, and at 12000μW / cm 2 Under the irradiation of a special ultraviolet lamp, react in an ice-water bath for 6 hours to obtain an intermediate solution of α-epoxy-ω-hydroxyl;
[0041] S2: Add diphenylmethane diisocyanate to the above intermediate solution, the molar ratio of diphenylmethane diisocyanate to the intermediate is 1:1, turn on the stirring device, raise the temperature to 50°C, and continue the reaction for 12 hours. After the end, the solvent was removed from the reaction product under the action of a rotar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com