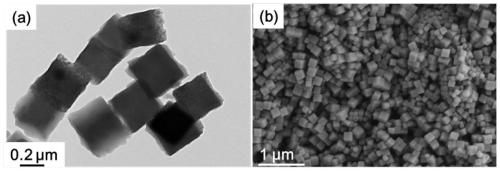
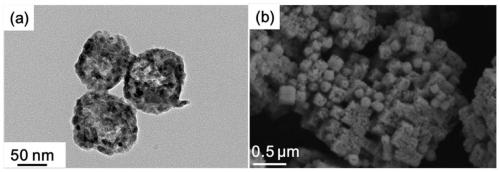
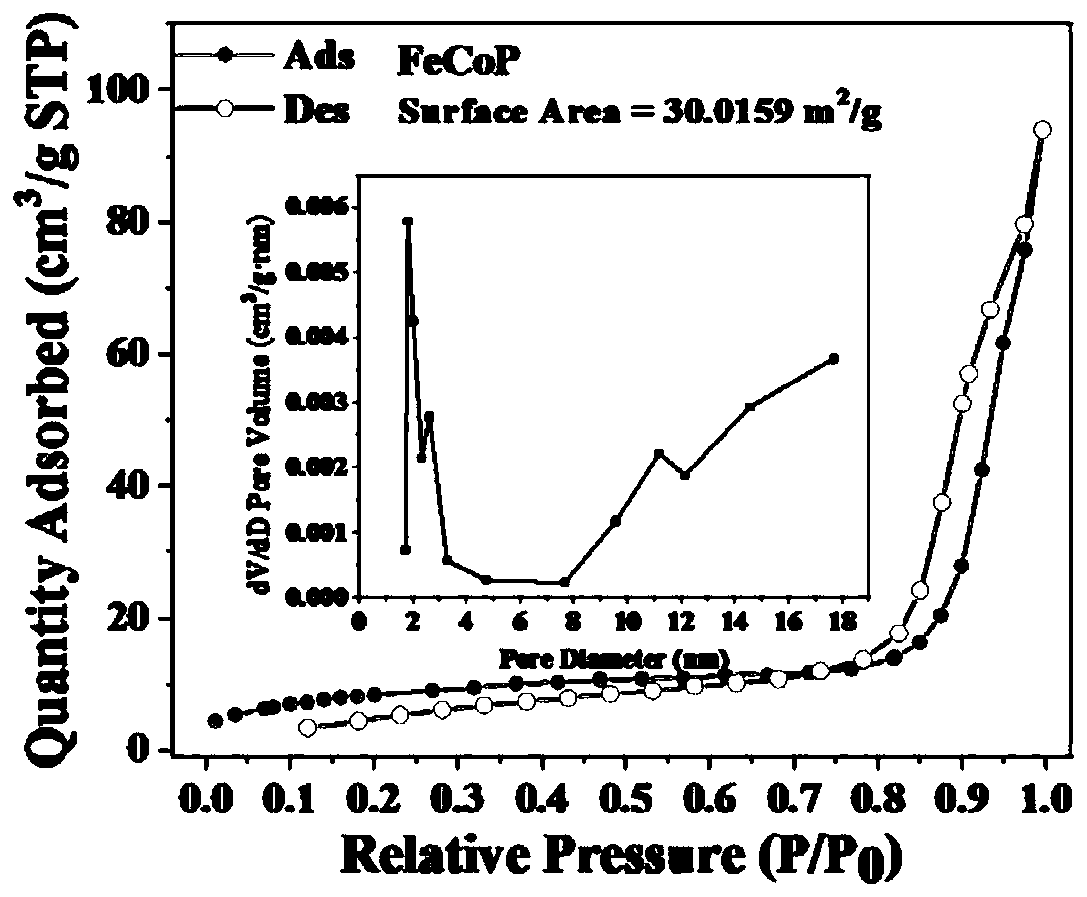
Porous cubic bimetallic phosphide catalyst as well as preparation method and application thereof
A cubic, bimetallic technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve problems such as slow kinetics, achieve good stability, abundant active sites, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] A preparation method of a porous cubic bimetallic phosphide catalyst, comprising the following steps:
[0042] Step S1: Preparation of Solution A
[0043] Add a deflocculant and a complexing stabilizer to deionized water, and after ultrasonic stirring and dissolving, add a transition metal salt to it, and stir evenly to obtain a solution A, the concentration of the deflocculating agent and the complexing stabilizer in the solution A is 1 -300mmol / L, the concentration of transition metal salt is 0.5-300mmol / L;
[0044] Step S2: Preparation of Solution B
[0045] Add transition metal potassium cyanide into deionized water, and stir to obtain solution B, wherein the concentration of transition metal potassium cyanide is 0.3-300mmol / L;
[0046] Step S3: Preparation of Solution C
[0047] Mix solution A and solution B obtained in step S1 and step S2, and ultrasonically stir for 5-10 minutes at a temperature of 298-313K to obtain solution C;
[0048] Step S4: Preparation ...
Embodiment 1
[0057] A preparation method of a porous cubic bimetallic phosphide catalyst, comprising the following steps:
[0058] Step S1: Preparation of Solution A
[0059] Add 1.5 mmol of sodium citrate to 60 ml of deionized water, and after ultrasonically stirring to dissolve, add 1.5 mmol of cobalt chloride hexahydrate to it, and stir to obtain solution A;
[0060] Step S2: Preparation of Solution B
[0061] Add 1.0 mmol potassium ferricyanide to 60 ml of deionized water, stir well to obtain solution B;
[0062] Step S3: Preparation of Solution C
[0063] Mix solution A and solution B obtained in step S1 and step S2, and ultrasonically stir for 10 min at a temperature of 298K to obtain solution C;
[0064] Step S4: Preparation of Prussian blue analogs
[0065] The solution C obtained in step S3 was left to age for 24 hours, centrifuged to obtain a precipitated product, washed 3 times with absolute ethanol, and dried in an oven at a temperature of 333K to obtain an iron-cobalt Prus...
Embodiment 2
[0069] A preparation method of a porous cubic bimetallic phosphide catalyst, comprising the following steps:
[0070] Step S1: Preparation of Solution A
[0071] Add 1.5 mmol of sodium tartrate to 60 ml of deionized water, and after ultrasonically stirring to dissolve, add 1.5 mmol of nickel carboxylate to it, and stir to obtain solution A;
[0072] Step S2: Preparation of Solution B
[0073] Add 1.0mmol potassium cobaltcyanide to 60ml deionized water, stir well to obtain solution B;
[0074] Step S3: Preparation of Solution C
[0075] Mix solution A and solution B obtained in step S1 and step S2, and ultrasonically stir for 8 minutes at a temperature of 303K to obtain solution C;
[0076] Step S4: Preparation of Prussian blue analogs
[0077] The solution C obtained in step S3 was left to age for 36 hours, centrifuged to obtain a precipitated product, washed 4 times with absolute ethanol, and dried in an oven at a temperature of 333K to obtain an iron-cobalt Prussian blue...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com