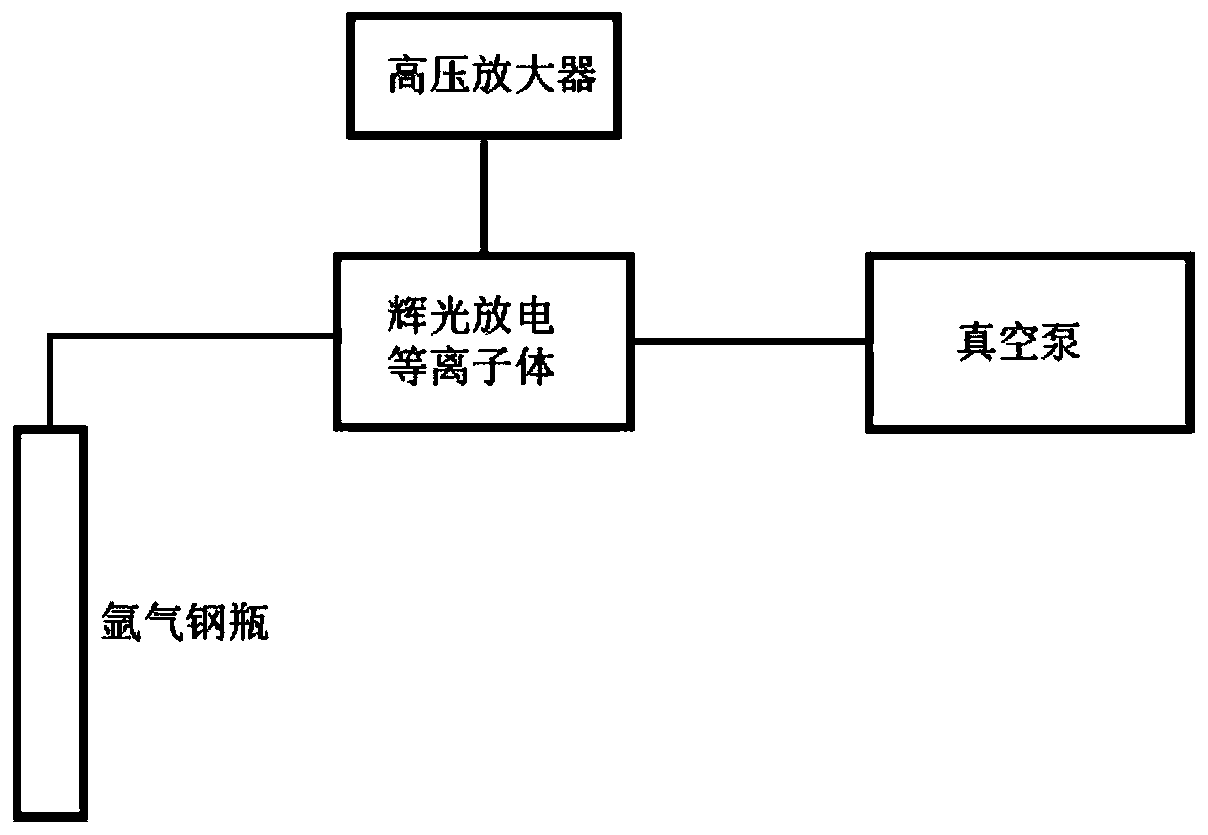
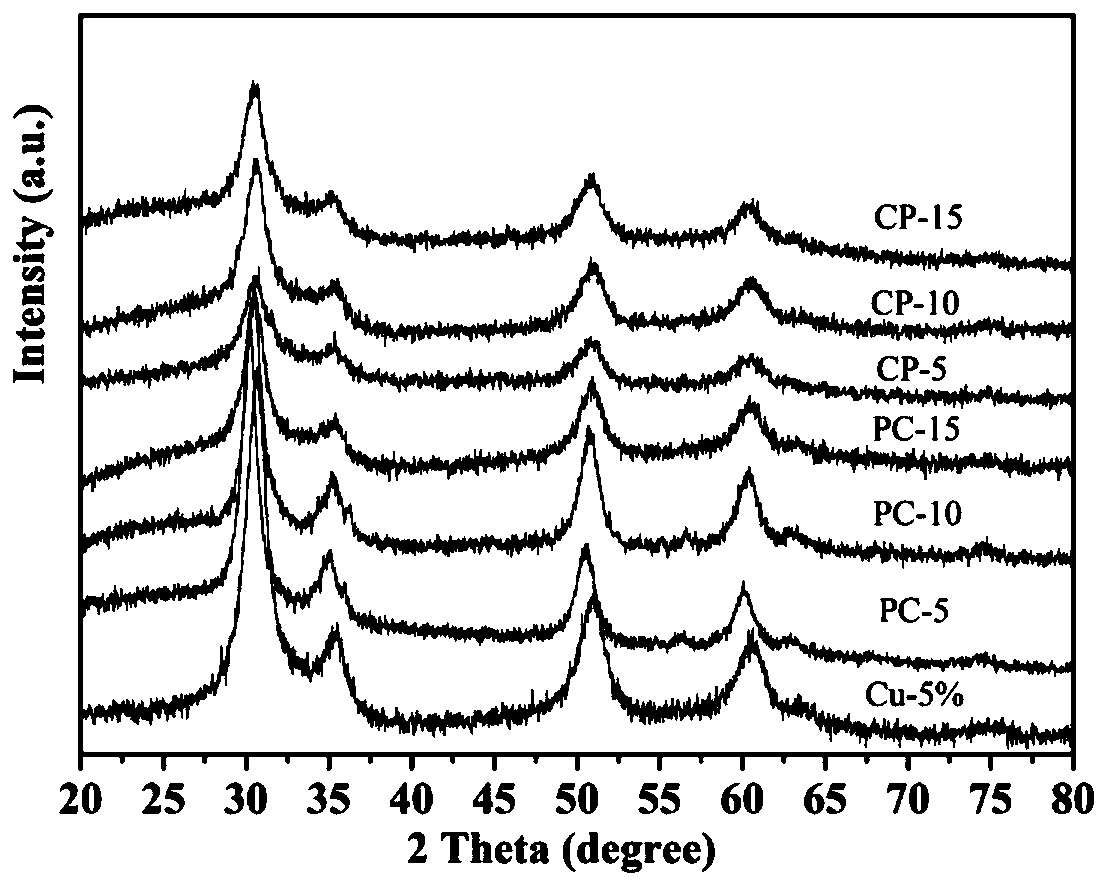
Cu/ZnO-ZrO2 solid solution catalyst and glow discharge plasma assisted preparation method and application thereof
A plasma and glow discharge technology, applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., can solve the problem of easy sintering of active components, poor methanol selectivity, Low conversion rate of carbon dioxide and other problems, to achieve the effect of inhibiting sintering phenomenon, high catalytic activity and high activity dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Weigh Zn(NO 3 ) 2 ·6H 2 O 1.49g, Zr(NO 3 ) 4 ·5H 2 O 8.59g Cu(NO 3 ) 2 ·3H 2 O 0.32g was dissolved in deionized water to form a metal nitrate mixed solution, and 26.50g of anhydrous sodium carbonate was weighed to prepare Na with a concentration of 0.5mol / L 2 CO 3 solution;
[0051] (2) At a reaction temperature of 70°C, slowly drop the above solution in parallel in a three-necked flask, and at the same time perform magnetic stirring to control the pH value of the solution at the end point of the titration to be about 7.5. After the titration is completed, continue stirring for 1 hour and then stop stirring. Stand still at the reaction temperature for 3 hours, centrifugally filter the solution at a rotating speed of 12000r / min, add deionized water to wash 3 times, and obtain a solid precipitate;
[0052] (3) drying in a vacuum oven at 105° C. for 12 hours to obtain a copper-doped zinc-zirconium solid solution catalyst precursor;
[0053] (4) Grind the obt...
Embodiment 2
[0056] (1) Weigh Zn(NO 3 ) 2 ·6H 2 O 1.49g, Zr(NO 3 ) 4 ·5H 2 O 8.59g Cu(NO 3 ) 2 ·3H 2 O 0.32g was dissolved in deionized water to form a metal nitrate mixed solution, and 26.50g of anhydrous sodium carbonate was weighed to prepare Na with a concentration of 0.5mol / L 2 CO 3 solution;
[0057] (2) At a reaction temperature of 70°C, slowly drop the above solution in parallel in a three-necked flask, and at the same time perform magnetic stirring to control the pH value of the solution at the end point of the titration to be about 7.5. After the titration is completed, continue stirring for 1 hour and then stop stirring. Stand still at the reaction temperature for 3 hours, centrifugally filter the solution at a rotating speed of 12000r / min, add deionized water to wash 3 times, and obtain a solid precipitate;
[0058] (3) drying in a vacuum oven at 105° C. for 12 hours to obtain a copper-doped zinc-zirconium solid solution catalyst precursor;
[0059] (4) Grind the obt...
Embodiment 3
[0062] (1) Weigh Zn(NO 3 ) 2 ·6H 2 O 1.49g, Zr(NO 3 ) 4 ·5H 2 O 8.59g Cu(NO 3 ) 2 ·3H 2 O 0.32g was dissolved in deionized water to form a metal nitrate mixed solution, and 26.50g of anhydrous sodium carbonate was weighed to prepare Na with a concentration of 0.5mol / L 2 CO 3 solution;
[0063] (2) At a reaction temperature of 70°C, slowly drop the above solution in parallel in a three-necked flask, and at the same time perform magnetic stirring to control the pH value of the solution at the end point of the titration to be about 7.5. After the titration is completed, continue stirring for 1 hour and then stop stirring. Stand still at the reaction temperature for 3 hours, centrifugally filter the solution at a rotating speed of 12000r / min, add deionized water to wash 3 times, and obtain a solid precipitate;
[0064] (3) drying in a vacuum oven at 105° C. for 12 hours to obtain a copper-doped zinc-zirconium solid solution catalyst precursor;
[0065] (4) Grind the obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com