A kind of synthetic method of promestriene
A synthesis method and technology of prostalene, applied in steroids, organic chemistry and other directions, can solve the problems of intractable treatment, difficult control of metal sodium, and difficult post-processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
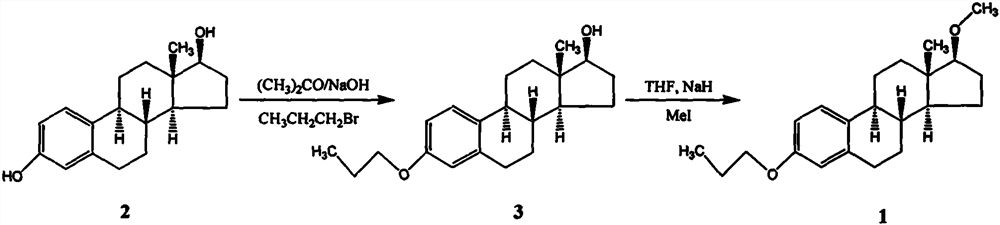
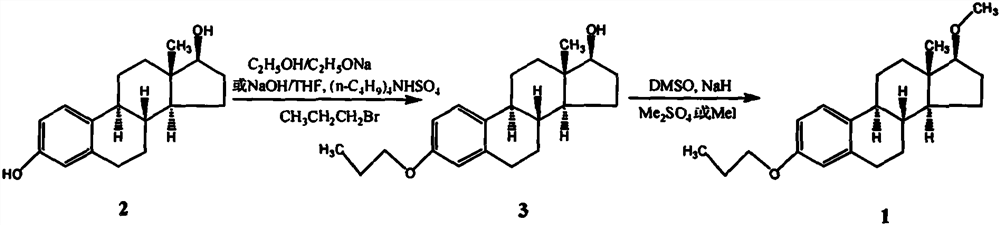
[0023] Synthesis of 3-propoxy-17β-hydroxyestr-1,3,5(10)-triene
[0024] Add 40 g of estradiol and 400 ml of acetone into a 1000 ml three-necked flask, stir and dissolve, and then add 8.8 g of sodium hydroxide to the solution. After stirring for 30 minutes, 36 g of bromopropane was added to the above reaction liquid, slowly heated to reflux, and refluxed for 3 hours. After the completion of the reaction as monitored by TLC, the heating was stopped, and the solvent was distilled off under reduced pressure. The residue was dissolved in 400 ml of ethyl acetate, washed with water until neutral, and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure, and the residue was completely dissolved in 150ml of anhydrous methanol. After natural cooling, crystals were precipitated, filtered, and the filter cake was washed twice with cold anhydrous methanol, and vacuum-dried at 60°C to obtain white crystals of 3-propoxy- 42.8 g of 17β-hydroxyestro-1,3,5(10)-t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com