Salts and polymorphs of pyrimidine compound, and pharmaceutical composition and preparation method and application of salts and polymorphs of pyrimidine compound
A kind of technology of compound and crystal form, applied in the field of salts and polymorphs of pyrimidine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Embodiment 1, hydrochloride crystal form III
[0152] 1 (31.21mg, 1.0eq) was dissolved in a mixed solvent of acetonitrile and dichloromethane (48v, 3 / 1), and hydrochloric acid (1.1eq) was added with stirring at 50°C. After the reaction solution was cooled to room temperature, it was stirred for 30 minutes. The resulting clear solution was then washed with N 2 The stream was concentrated to about 32v and a solid precipitated out immediately. The resulting suspension was stirred overnight at room temperature, and the solid was collected by filtration and dried in vacuo at 50° C. for about 4 hours to obtain the hydrochloride salt form III. The sample is off-white solid, and XRPD, DSC, TGA, DVS and 1 H-NMR characterization.
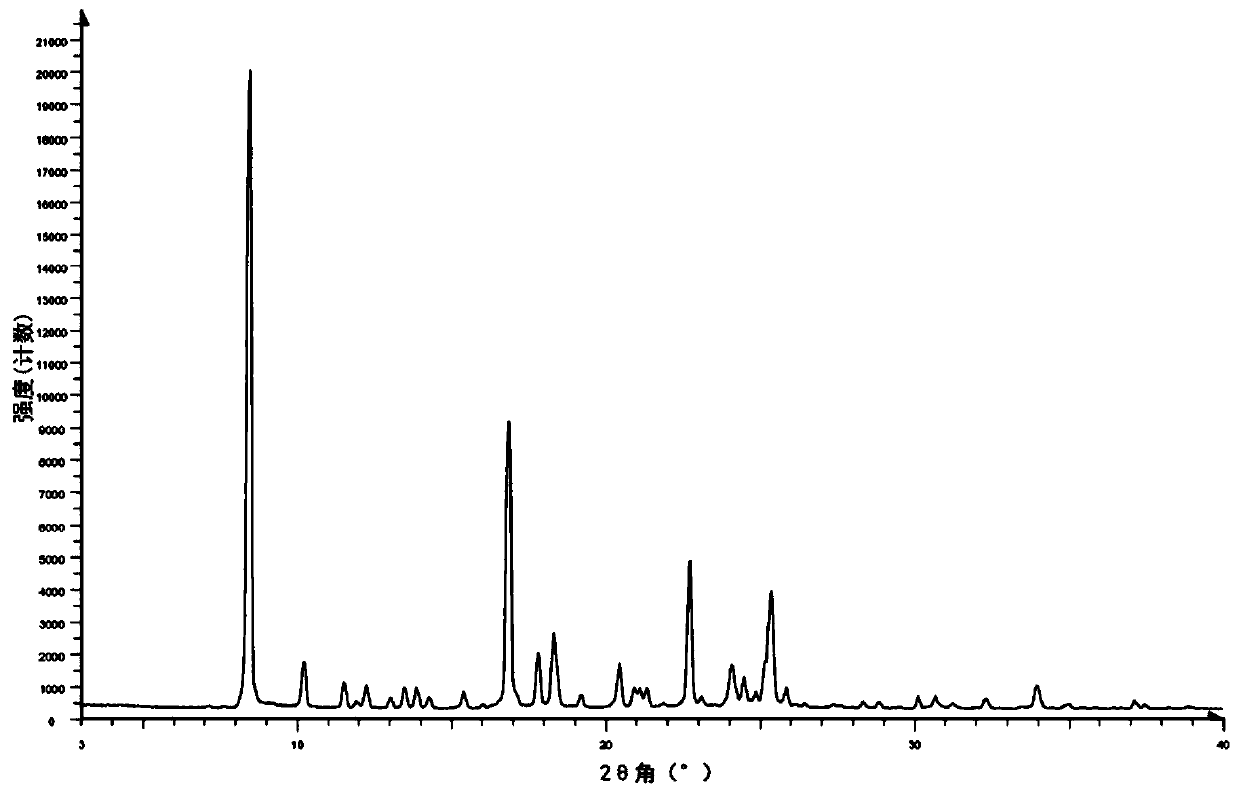
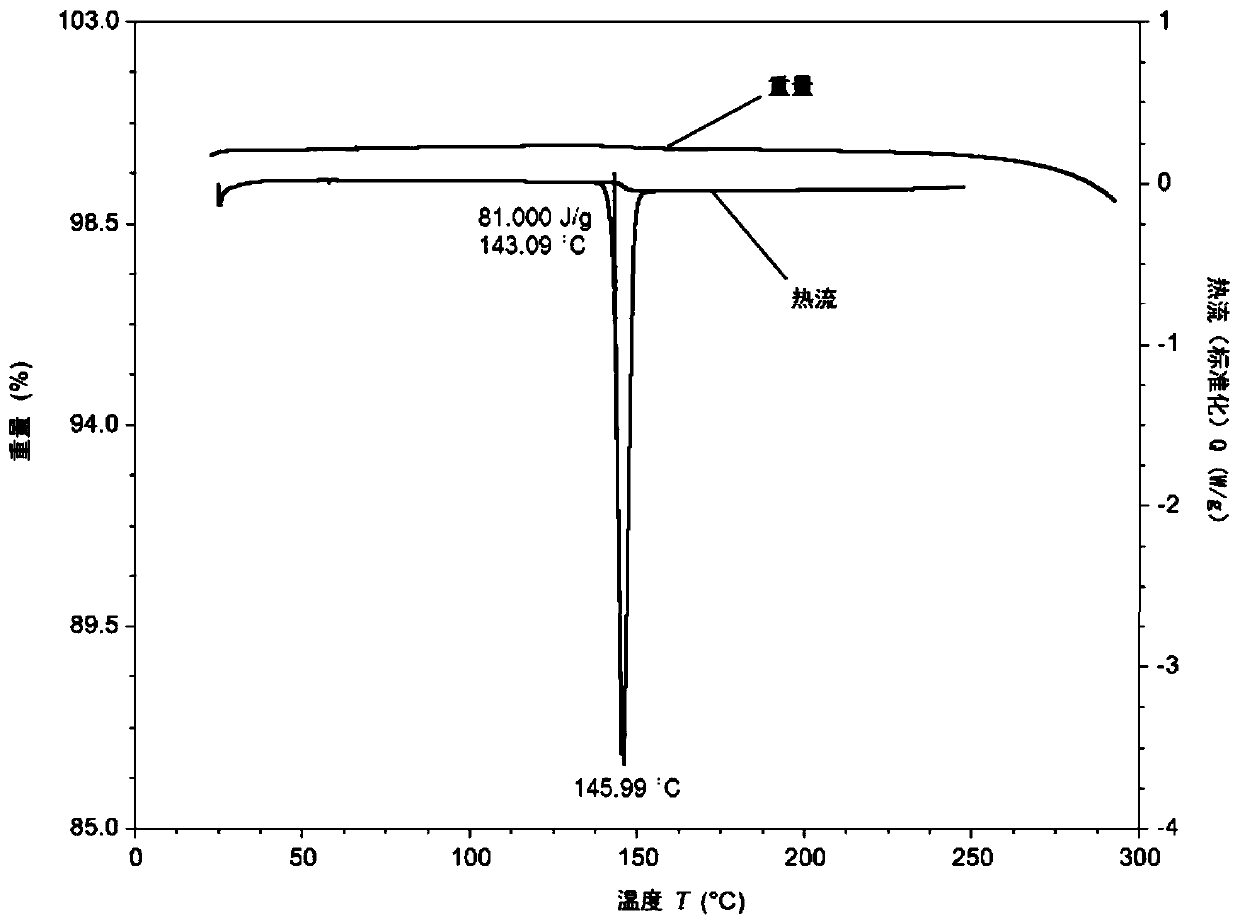
[0153] The hydrochloride salt form III has a high melting point (273°C, Figure 5 ) crystals (Table 1 and Figure 4 ). The sample has slight hygroscopicity, and the weight gain is about 1.86% under the condition of 80% relative humidity ( Figu...
Embodiment 2
[0157] Embodiment 2, phosphate crystal form I
[0158] 1 (30.20mg, 1.0eq) was dissolved in acetone (26v). After adding phosphoric acid (1.1eq) under stirring at room temperature, sticky matter was precipitated immediately, and a solid precipitated after continued stirring for 2 hours. After the suspension was stirred at room temperature for 3 hours, the solid was collected by filtration and vacuum-dried overnight at 50°C to obtain phosphate crystal form I. The sample was an off-white solid, and XRPD, DSC, TGA, DVS and 1 H-NMR characterization.
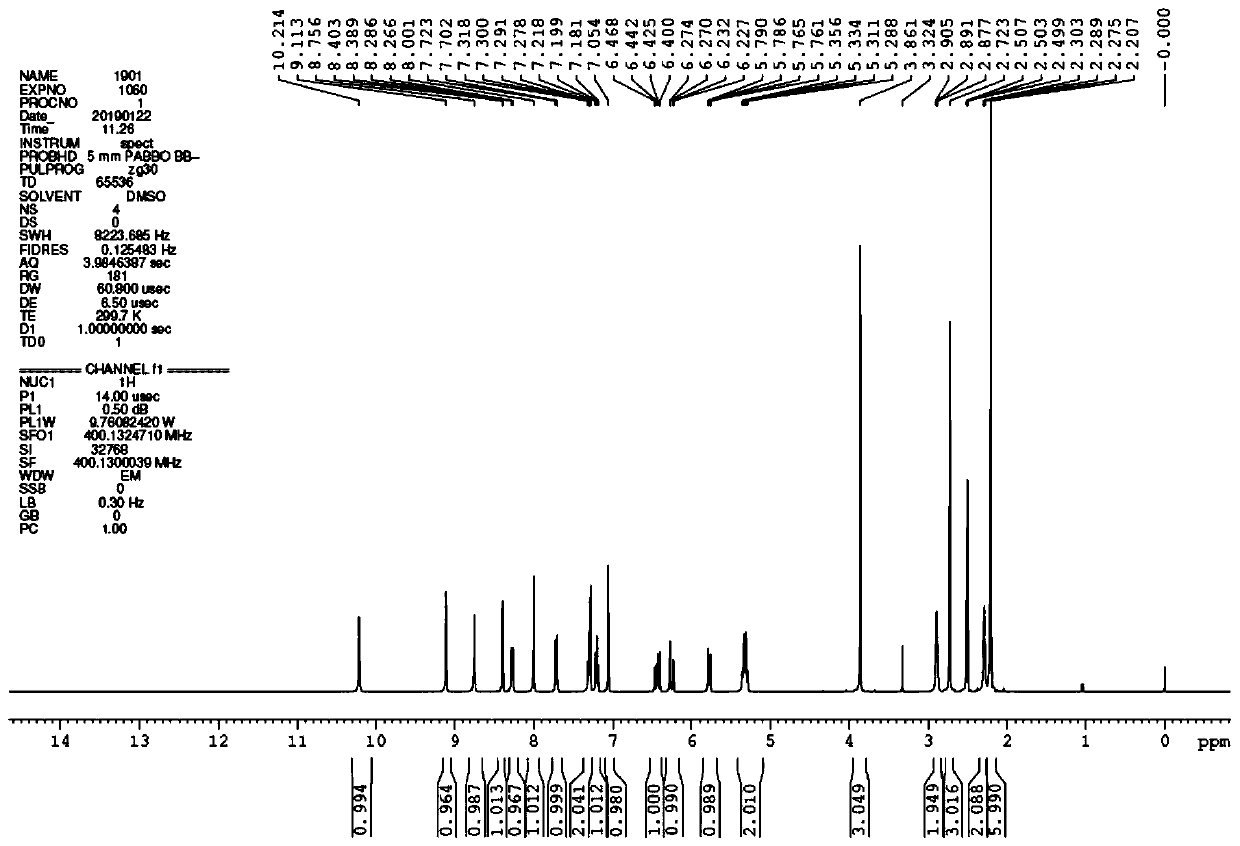
[0159] Phosphate crystal form I is a crystal with high crystallinity (Table 2 and Figure 9 ), high melting point (238°C, Figure 10 ) crystals. The sample is slightly hygroscopic, and its weight gain is about 0.61% ( Figure 12 ). 1 H-NMR and TGA results showed that the sample had 0.7% residual solvent, but no significant weight loss before 150°C ( Figure 10 and Figure 11 ), indicating that the sample is anhydrous. After the...
Embodiment 3
[0163] Example 3, Form I of p-toluenesulfonate salt
[0164] 1 (31.60mg, 1.0eq) was dissolved in acetone (25v), and p-toluenesulfonic acid (1.1eq) was added with stirring at room temperature. After about 2 minutes, a solid precipitated out. The suspension continued to stir at room temperature for about 6 hours. The solid was collected by filtration and dried in vacuo at 50°C overnight to obtain Form I of p-toluenesulfonate salt. The sample was an off-white solid, and XRPD, DSC, TGA, DVS and 1 H-NMR characterization.
[0165] Form I of p-toluenesulfonate salt has a melting point of 172°C ( Figure 15 ) crystals (Table 3 and Figure 14 ). The sample is slightly hygroscopic, with a weight gain of about 0.55% ( Figure 17 ). TGA showed that the sample had no obvious weight loss before 200°C ( Figure 15 ); 1 H-NMR shows that the sample has about 0.3% residual solvent, and the ratio of free base and p-toluenesulfonic acid is 1:1 ( Figure 16 ). This sample is probably anh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com