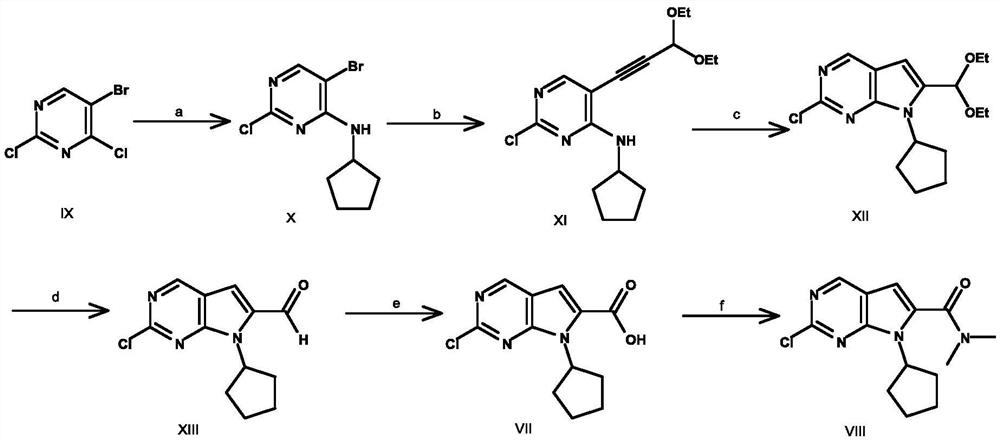
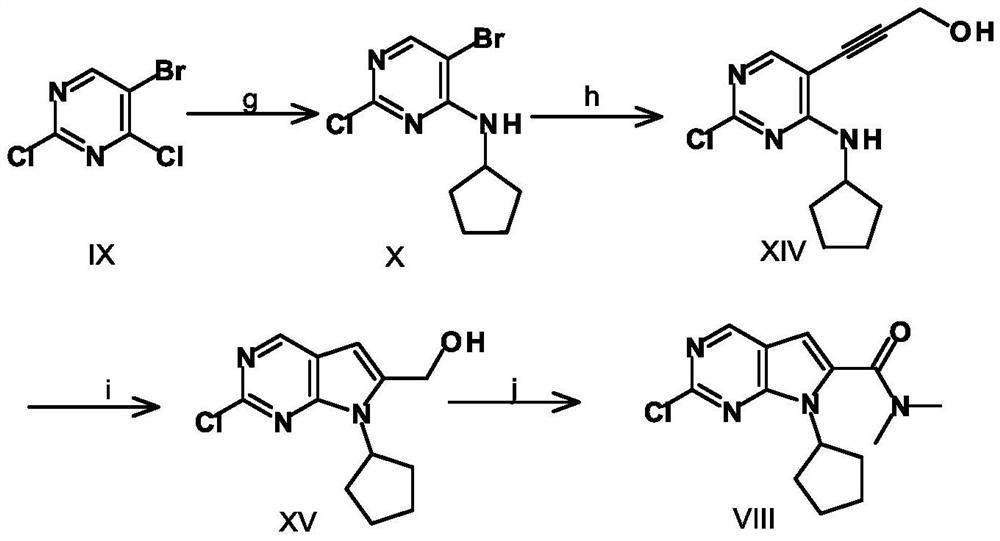
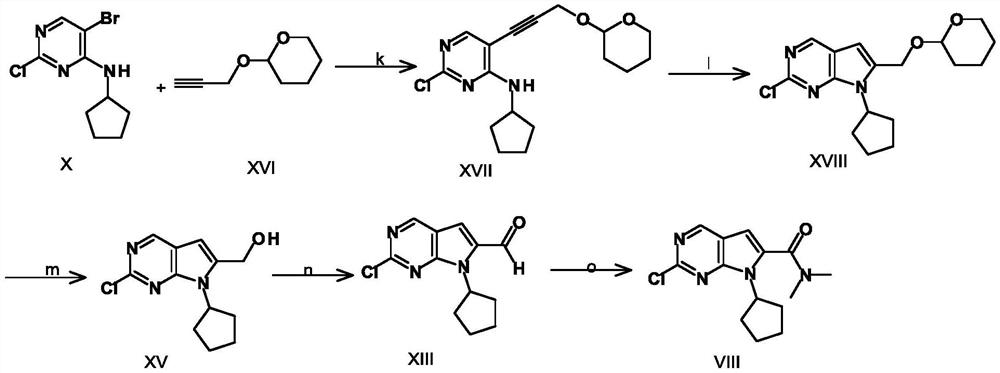
A key intermediate for synthesizing cdk4/6 dual inhibitor and its preparation method and application
A compound and reaction temperature technology, applied in organic chemistry, bulk chemical production, etc., can solve problems such as expensive, difficult to obtain raw material compound XVI, and not environmentally friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation of compound III-1:
[0059]
[0060] In a 50L double-layer kettle, add compound II (2500g, 13.3mol, 1.0e.q.) into 20L of dichloromethane, add triethylamine (2019g, 19.95mol, 1.5e.q.), after the addition is complete, add benzenesulfonate dropwise at 0°C Acyl chloride (2584g, 14.63mol, 1.1e.q.), temperature controlled 0-10°C, the dropwise addition was completed, stirred at 10°C for 5h, and LC-MS detected that the reaction was complete. Add 25L of water to the reaction solution, stir well and separate the liquids, wash the organic phase with 25L of saturated sodium bicarbonate solution, wash with 25L of water and separate the liquids, add anhydrous magnesium sulfate to dry, filter, evaporate the solvent under reduced pressure, and use Slurry with petroleum ether and dry to obtain 3675.0 g of compound III-1 as a light yellow solid with a yield of 84.2%. 1 HNMR (400MHz, CDCl 3 , δppm) 8.26-8.25 (d, 2H), (7.78-7.58, m, 4H), 6.72-6.71 (d, 1H).
[0061] Prepar...
Embodiment 2
[0073] Preparation of compound III-2:
[0074]
[0075] In a 50L double-layer kettle, compound II (2500g, 13.3mol, 1.0e.q.) was added to 30L THF, and at 0°C, NaH (797.8g, 19.95mol, 1.5e.q.) was added in batches. Add p-toluenesulfonyl chloride (3042g, 15.96mol, 1.2e.q.), control the temperature at 0-10°C, dropwise, stir at 20°C for 6h, and LC-MS detects that the reaction is complete. Add 35L of water to the reaction solution, stir well and separate the liquids, wash the organic phase with 30L of saturated sodium bicarbonate solution, wash with 30L of water and separate the liquids, add anhydrous magnesium sulfate to dry, filter, evaporate the solvent under reduced pressure, and use Slurry with petroleum ether and dry to obtain 3881.3 g of compound III-2 as a light yellow solid with a yield of 85.3%.
[0076] Preparation of Compound IV-2:
[0077]
[0078] Compound III-2 (2000g, 5.84mol, 1.0e.q.) in 10L tetrahydrofuran solution, cooled to -50°C, added dropwise 2.5M NaHMD...
Embodiment 3
[0089] Preparation of compound III-1:
[0090]
[0091] In a 50L double-layer kettle, compound II (2500g, 13.3mol, 1.0e.q.) was added to 20L of dichloromethane, and DBU (3035g, 19.95mol, 1.5e.q.) was added. After the addition was complete, benzenesulfonyl chloride ( 2819g, 15.96mol, 1.2e.q.), the temperature was controlled at 0-10°C, the dropwise addition was completed, the reaction was stirred at 30°C for 5h, and the reaction was detected by LC-MS. Add 25L of water to the reaction solution, stir well and separate the liquids, wash the organic phase with 25L of saturated sodium bicarbonate solution, wash with 25L of water and separate the liquids, add anhydrous magnesium sulfate to dry, filter, evaporate the solvent under reduced pressure, and use Slurry with petroleum ether and dry to obtain 3723.1 g of compound III-1 as a light yellow solid with a yield of 85.3%. 1 HNMR (400MHz, CDCl 3 ), 8.26-8.25 (d, 2H), (7.78-7.58, m, 4H), 6.72-6.71 (d, 1H).
[0092] Preparation of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com