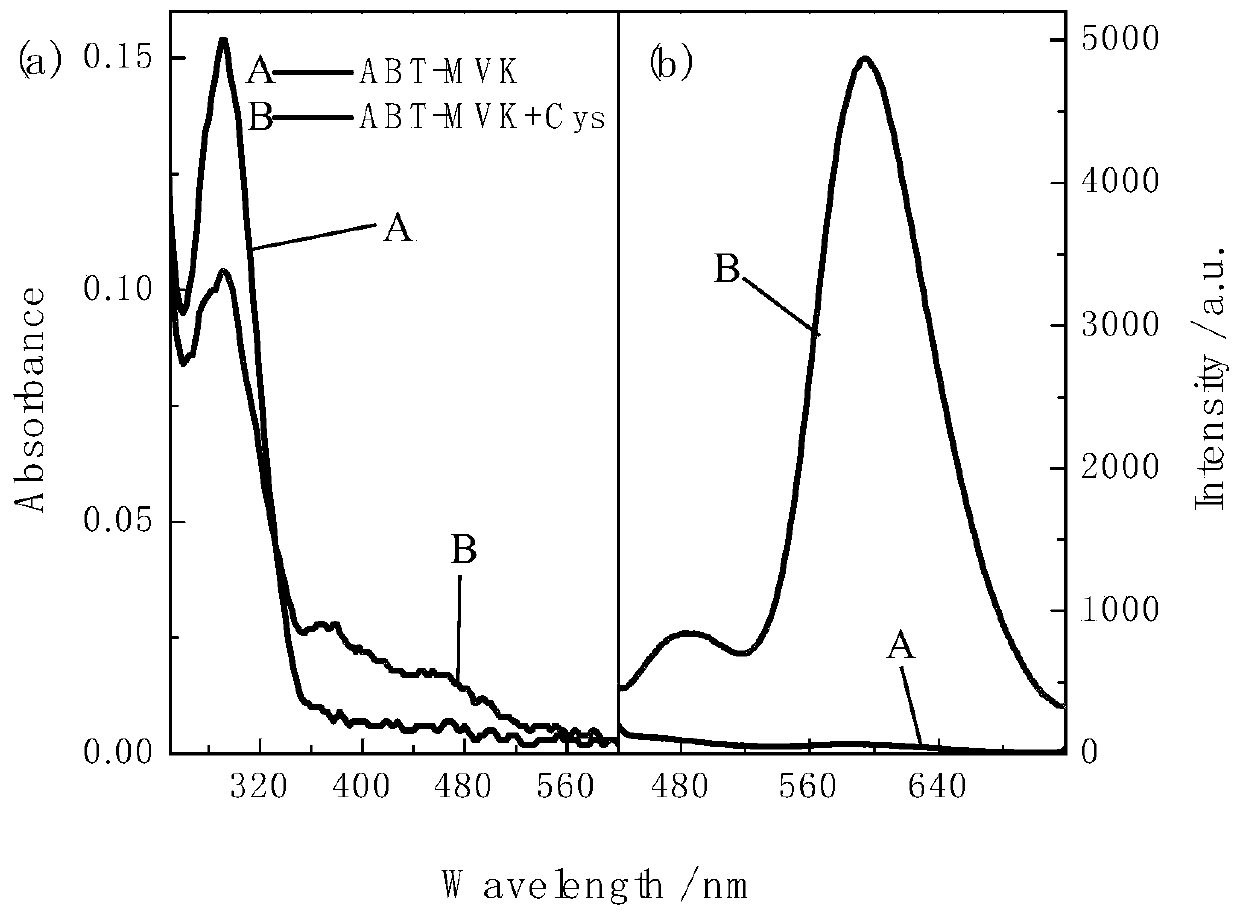
Fluorescent probe with large Stokes shift as well as synthesis method and application of fluorescent probe
A technology of fluorescent probes and synthesis methods, applied in fluorescence/phosphorescence, chemical instruments and methods, organic chemistry, etc., to achieve the effects of good research value, simple operation, and convenient purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
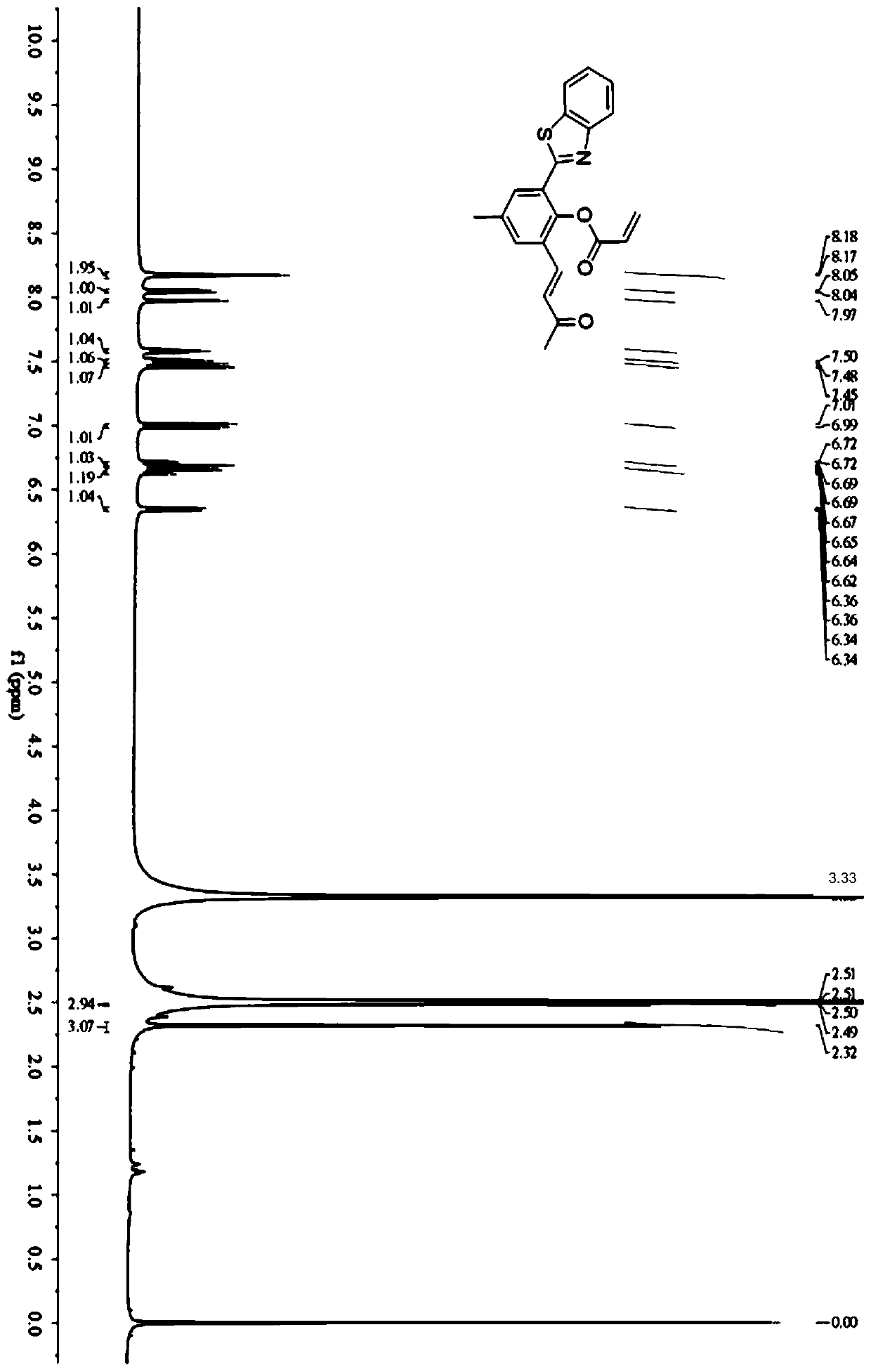
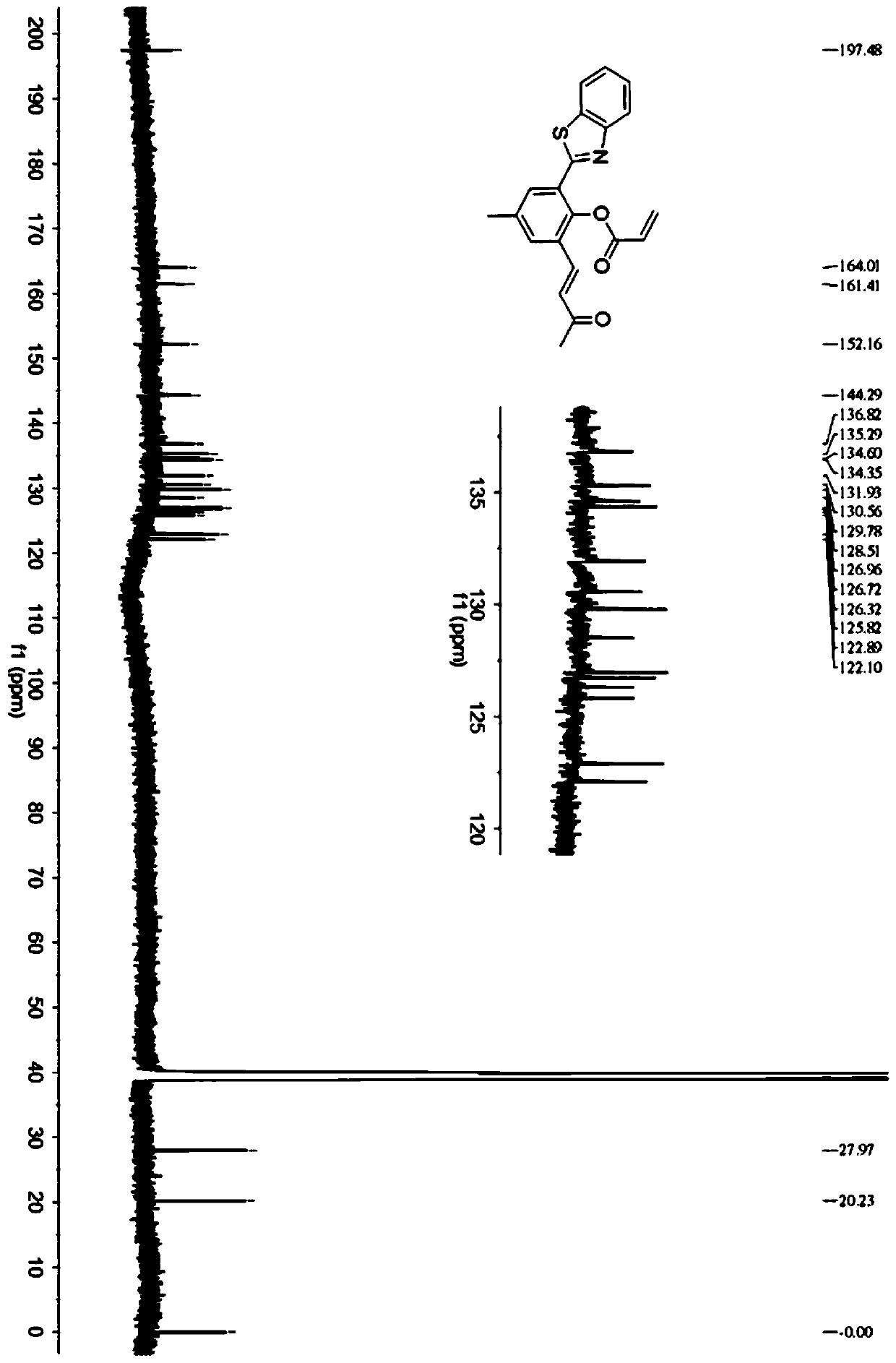
[0057] The invention also discloses a method for synthesizing a fluorescent probe with a large Stokes shift, and the specific steps include:
[0058] (1) Dissolve 2-aminothiophenol, 5-methyl salicylaldehyde and iodine in methanol, stir and react at room temperature for 3-5 hours, and obtain compound 1 by suction filtration;
[0059] (2) Dissolving compound 1 and hexamethylenetetramine in trifluoroacetic acid, heating and refluxing at 105°C to 115°C for 22 to 24 hours, and adjusting the pH to neutral, precipitated solid, and filtered to obtain compound 2;
[0060] (3) Dissolve compound 2 in acetone, heat to 45°C-55°C, then slowly add alkali solution dropwise until the solution turns red, continue heating for 3-5h to obtain compound 3;
[0061] (4) Compound 3 was dissolved in CH containing triethylamine 2 Cl 2 , stirring at low temperature, while slowly adding acryloyl chloride to react, and moving the reaction solution to room temperature and stirring for 3 to 5 hours, then t...
Embodiment 1
[0068] Synthesis of fluorescent probes with large Stokes shifts:
[0069] (1) Synthesis of compound 1:
[0070] 2-Aminothiophenol (10.0mmol), 5-methylsalicylaldehyde (10.0mmol) and iodine (5.0mmol), stirred at room temperature, obtained compound 1 by suction filtration; the solvent of the reaction was methanol; the reaction The time is 4h.
[0071] (2) Synthesis of compound 2:
[0072] Dissolve 0.432 g (2.0 mmol) of 2-(2-hydroxyphenyl)-benzothiazole (HBT) and 0.840 g (6.0 mmol) of hexamethylenetetramine in 20 mL of TFA, and store the contents at 110 °C Heated to reflux and stirred for 24 hours, then stopped heating and allowed to cool to room temperature; the solution was neutralized with 1N NaOH, and the resulting precipitate was filtered and washed with water; finally on a silica gel column (CH 2 Cl 2 and MeOH 100:1, v / v) to give pure product compound 2.
[0073] (3) Synthesis of compound 3:
[0074] Dissolve 0.400g (1.5mmol) of compound 2 in 20mL of acetone, heat to 5...
Embodiment 2
[0078] Synthesis of fluorescent probes with large Stokes shifts:
[0079] The difference between this embodiment and Example 1 is that the reaction time of Step 1 in Example 1 is replaced with 3 hours, and the remaining reactants and experimental parameters refer to Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com