High-generation dendritic polylysine and preparation method thereof
A polylysine and dendritic technology, applied in the field of high-generation dendritic polylysine and its preparation, can solve the problem of low purity, poor drug loading and slow-release ability, and small internal space of molecules in high-generation dendritic polymers and other problems, to achieve the effect of excellent water solubility, wide application range and low manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044]
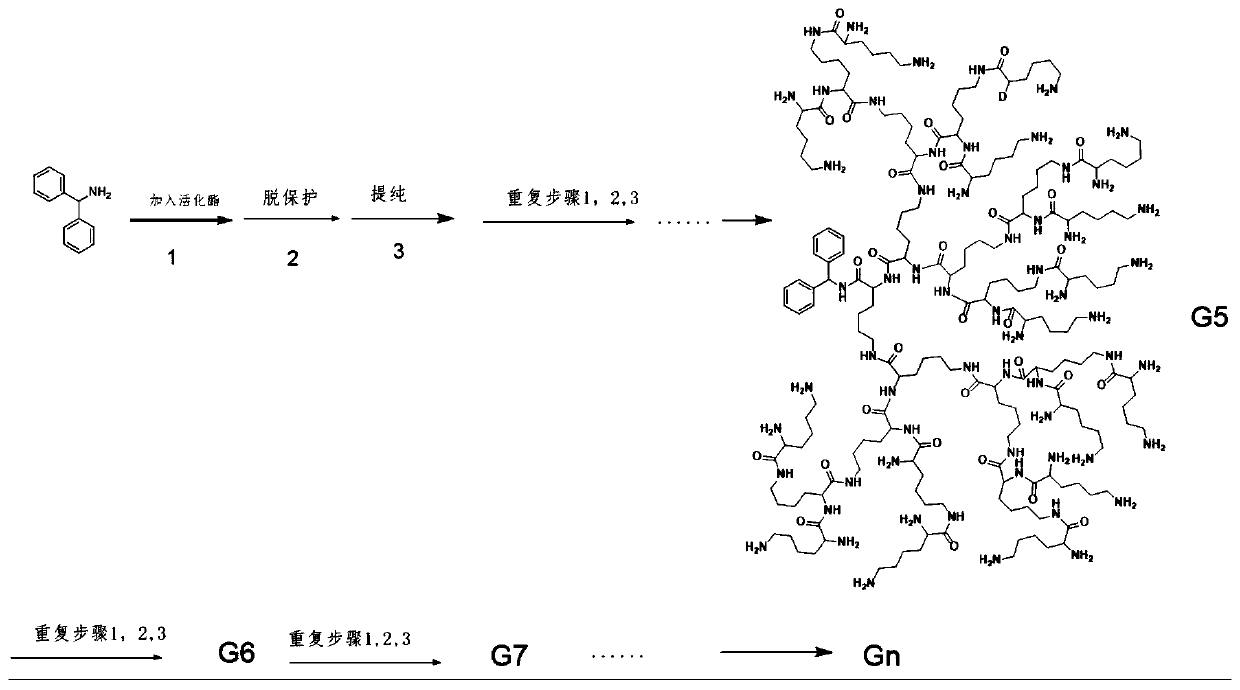
[0045] The preparation method of high-generation dendritic polylysine, it comprises the steps:
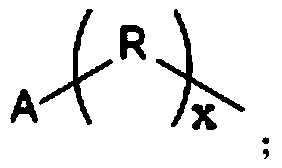
[0046] (1), react the lysine monomer containing diamino protection, N,N'-dicyclohexylcarbodiimide (DCC) and N-hydroxysuccinimide (HoSu) in an organic solvent to obtain lysine Amino acid activated ester monomer, its structural formula is:
[0047]
[0048] Wherein, the protecting group of diamino in the lysine monomer containing diamino protection is the same, Z represents the first amino protecting group, and Z' represents the second amino protecting group;
[0049] (2), react the lysine activated ester monomer of step (1) and the hydrophobic molecule containing amino group in organic solvent, obtain the n generation dendritic polylysine, its structural formula is:
[0050]
[0051] Wherein, n is an integer selected from 1-19, A represents a hydrophobic group, Z represents a first amino protecting group, and Z' represents a second amino protecting group;
[0052]...
Embodiment 1
[0076] The preparation method of the fifth generation dendritic polylysine containing benzyloxycarbonyl protection of the present embodiment comprises the following steps:
[0077] (1) 2.07g (0.005mol) of lysine containing two benzyloxycarbonyl groups (structure shown below), 1.03g of N,N'-dicyclohexylcarbodiimide and 0.69g of N-hydroxysuccinimide The amine was dissolved in 50ml of tetrahydrofuran, and reacted at 25°C for 10 hours to obtain the first lysine activated ester monomer;
[0078]
[0079] (2) After mixing 0.915g of benzhydrylamine and the reaction solution of the first lysine activated ester monomer, react at 25°C for 16h to obtain the first generation dendritic polylysine containing benzyloxycarbonyl protection;
[0080] (3) the dendritic polylysine containing benzyloxycarbonyl protection of the first generation in step (2) is dissolved in the acetic acid solution (as deprotecting agent) containing 30wt% hydrobromic acid (HBr) in 50ml, at 25 After reacting at ℃...
Embodiment 2
[0093] The preparation method of the fifth generation dendritic polylysine containing N-fluorenyl methaneoxycarbonyl protection comprises the following steps:
[0094] (1) Add 2.95g (0.005mol) of lysine containing two N-fluorenyl methaneoxycarbonyl groups (the structure is shown below), 1.03g of N,N'-dicyclohexylcarbodiimide and 0.69g of N-hydroxy Succinimide was dissolved in 50ml of THF, and reacted at 25°C for 8h to obtain the first lysine activated ester monomer;
[0095]
[0096] (2) After mixing 0.915g of diphenylmethylamine and the first lysine activated ester monomer reaction solution, react at 25°C for 16h to obtain the first generation dendrimer containing N-fluorenyl moxycarbonyl protection. Lysine;
[0097] (3) the dendritic polylysine that the first generation contains N-fluorenmoxycarbonyl protection in step (2) is dissolved in the dichloromethane solution that is 50% piperidine by volume fraction in 50ml (as deprotecting agent ), reacted at 25°C for 3 hours,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com