Compound ceftiofur hydrochloride breast injectant
A technology of ceftiofur hydrochloride and udder injection, which is applied to the prescription and preparation field of compound ceftiofur hydrochloride udder injection for dairy cows, and can solve the problem that the drug cannot be guaranteed to reach the udder, the therapeutic effect cannot be achieved, and the drug distribution is uneven, etc. To achieve the effect of enriching traditional Chinese medicine resources, reducing antibiotic residues and drug resistance and other hazards, and inhibiting Staphylococcus aureus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) the prescription of every 100ml compound ceftiofur hydrochloride breast injection is as follows:
[0033]
[0034]
[0035] 2) Ceftiofur hydrochloride was pretreated by airflow micronization technology to obtain ceftiofur hydrochloride micropowder with a particle size of 5 μm.
[0036] 3) The non-aqueous solvent (soybean oil for injection) with 90% of the prescription amount is filtered, and the treated non-aqueous solvent is heated to 110° C. for later use.
[0037] 4) Add suspending agent hydrogenated castor oil and surfactant soybean lecithin into the above non-aqueous solvent until completely dissolved.
[0038] 5) When the temperature is cooled to 35°C, add the prescribed amount of ceftiofur hydrochloride; when the temperature continues to cool to 30°C, add the volatile oil of forsythia, and then add filtered non-aqueous solvent to 100ml.
[0039] 6) Process the prepared above solution with a high-speed disperser, and shear at 6000r / min for 20min.
[004...
Embodiment 2
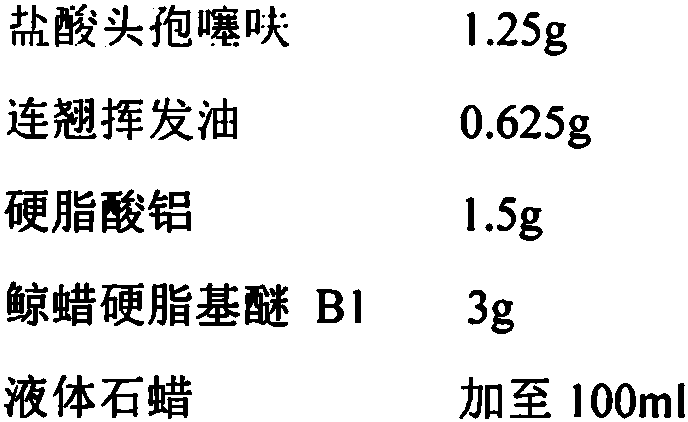
[0042] 1) The prescription of compound ceftiofur hydrochloride breast injection per 100ml is as follows:
[0043]
[0044] 2) Ceftiofur hydrochloride was pretreated by airflow micronization technology to obtain ceftiofur hydrochloride micropowder with a particle size of 5 μm.
[0045] 3) Take 90% of the prescription amount of non-aqueous solvent (liquid paraffin) for filtration treatment, heat the treated non-aqueous solvent to 120° C., and set aside.
[0046] 4) Add the suspending agent aluminum stearate and the surfactant cetearyl ether B1 into the non-aqueous solvent until completely dissolved.
[0047] 5) When the temperature is cooled to 35°C, add the prescribed amount of ceftiofur hydrochloride; when the temperature continues to cool to 30°C, add the volatile oil of forsythia, and then add filtered non-aqueous solvent to 100ml.
[0048] 6) Process the prepared above solution with a high-speed disperser, and shear at 8000r / min for 20min.
[0049] 7) Filling and steri...
Embodiment 3
[0051] The prescription of compound ceftiofur hydrochloride breast injection per 100ml is as follows:
[0052]
[0053] 1) Get 90% of the non-aqueous solvent (soybean oil for injection) of the prescription amount to filter, and heat the processed non-aqueous solvent to 100°C for subsequent use.
[0054] 2) Add suspending agent hydrogenated castor oil and surfactant cetearyl ether B2 into the non-aqueous solvent until completely dissolved.
[0055] 3) When the temperature is cooled to 35°C, add the prescribed amount of ceftiofur hydrochloride; when the temperature continues to cool to 30°C, add the volatile oil of forsythia, and then add the filtered non-aqueous solvent to 100ml.
[0056] 4) Process the prepared above solution with a high-speed disperser, and shear at 10000r / min for 20min.
[0057] 5) Filling and sterilizing to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com