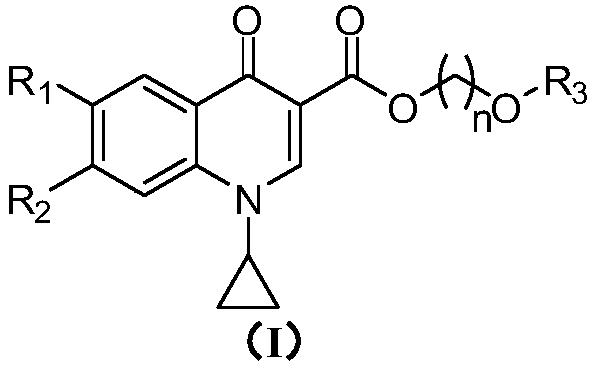
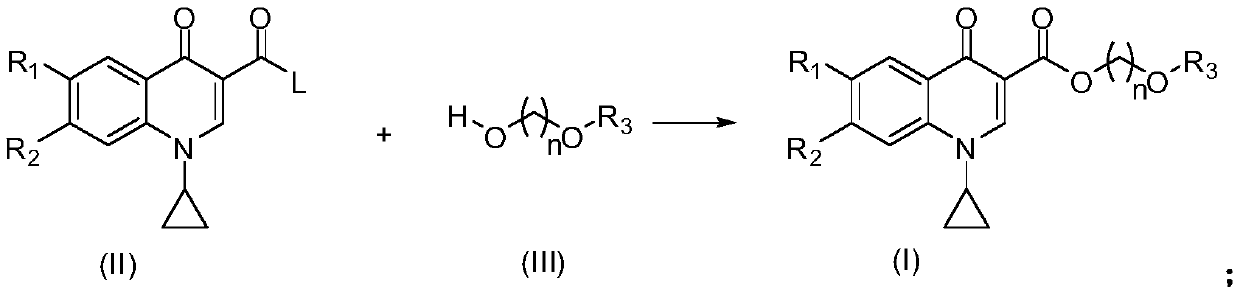
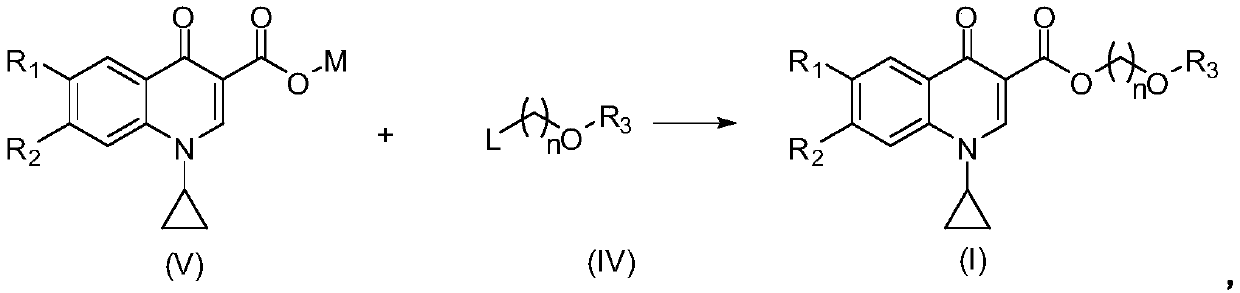
Quinoline carboxylic acid ester compound and preparation method and application thereof
A technology of ester compound and quinoline carboxylic acid is applied in the field of agricultural bactericide, which can solve the problems of small promotion area, limitation of resistance and control effect, pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] Example 1: Methoxymethyl 1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (compound 1)
[0151]
[0152] The first step reaction: Potassium 1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate
[0153] At room temperature, 1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (12.3g, 0.05mol), 30% aqueous potassium hydroxide solution (12ml) was added to In a three-neck bottle. Heated to 60°C and reacted for 2 hours. Heating was stopped, and the product was dehydrated under reduced pressure to obtain 13.6 g of the product with a yield of 96%.
[0154] The second step reaction: Methoxymethyl 1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate
[0155] At room temperature, potassium 1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (2.85g, 0.01mol), chloromethyl methyl ether (0.96g, 0.012 mol) were successively dissolved in N,N-dimethylformamide (15ml). Heated to 100°C and reacted for 6 hours. Heati...
Embodiment 2
[0157] Example 2: 2-methoxyethyl 1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (compound 10)
[0158]
[0159] The first step reaction: 1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-oyl chloride
[0160] At room temperature, mix 1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (14.9g, 0.06mol), dichloroethane (90ml) and N,N - Dimethylformamide (0.2g) was added into a three-necked flask, and thionyl chloride (14.6g, 0.12mol) was added dropwise to the above mixture. Heat to reflux for 5 hours. Heating was stopped, and the solvent and residual thionyl chloride were removed under reduced pressure to obtain 14.6 g of the product with a yield of 92%.
[0161] The second step reaction: 2-methoxyethyl 1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate
[0162] At room temperature, 2-methoxyethanol (0.76g, 0.01mol) and triethylamine (2.02g, 0.02mol) were successively dissolved in dichloromethane (20ml). Cool to 0°C in a cry...
Embodiment 3
[0164] Example 3: 2-methoxyethyl 6-chloro-1-cyclopropyl-4-oxo-1,4-dihydroquinoline-3-carboxylate (compound 45)
[0165]
[0166] The first step reaction: 6-chloro-1-cyclopropyl-4-oxo-1,4-dihydroquinoline-3-acid chloride
[0167] At room temperature, mix 6-chloro-1-cyclopropyl-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (15.8g, 0.06mol), dichloroethane (90ml) and N,N - Dimethylformamide (0.2g) was added into a three-necked flask, and thionyl chloride (14.6g, 0.12mol) was added dropwise to the above mixture. Heat to reflux for 5 hours. Heating was stopped, and the solvent and residual thionyl chloride were removed under reduced pressure to obtain 16.2 g of the product with a yield of 96%.
[0168] The second step reaction: 2-methoxyethyl 6-chloro-1-cyclopropyl-4-oxo-1,4-dihydroquinoline-3-carboxylate
[0169] At room temperature, 2-methoxyethanol (0.76g, 0.01mol) and triethylamine (2.02g, 0.02mol) were successively dissolved in dichloromethane (20ml). Cool to 0°C in a cr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com