Cyclic peptide resistant to multi-drug resistant bacteria and preparation method and application thereof
A technology of multi-drug resistant bacteria and cyclic peptides, applied in the field of cyclic peptides, can solve the problems of identifying antimicrobial peptides, difficult and other problems, and achieve the effects of broad-spectrum killing activity, strong operability, and strong bactericidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
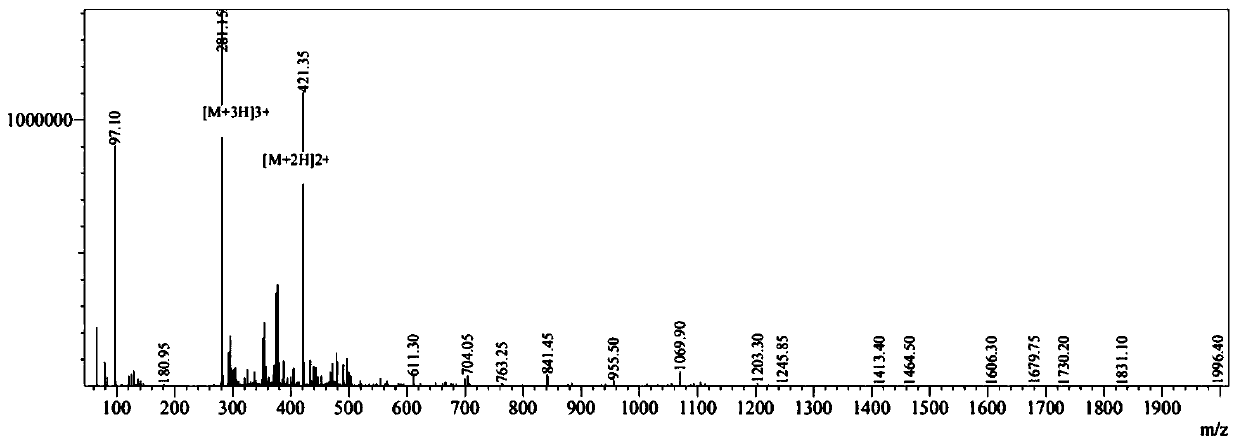
[0041] The synthesis of embodiment 1 antimicrobial peptide CPeptide-A:R-R-W-W-R (Arg-Arg-Trp-Trp-Arg)
[0042] Using the Fmoc solid-phase peptide synthesis method, using dichloro resin 2-CTC resin as a carrier, first synthesize a fully protected linear peptide, use a cleavage reagent to cleavage to obtain a fully protected linear peptide, and then cyclize in the liquid phase to obtain a fully protected cyclic peptide. After deprotection with trifluoroacetic acid, the primary product of cyclic peptide was obtained by ether precipitation.
[0043] The specific operation steps are:
[0044] (1) Resin activation
[0045] Weigh 1 g of resin and swell with 10 ml of DCM (dichloromethane) at room temperature for 30 min.
[0046] (2) The first amino acid is coupled to the resin
[0047] Weigh the protected amino acid Fmoc-Arg(Pbf)-OH with a total resin substitution value of 3eq and add it to the DCM solution, then add DIEA (N,N-diisopropylethylamine) with a total resin substitution ...
Embodiment 2
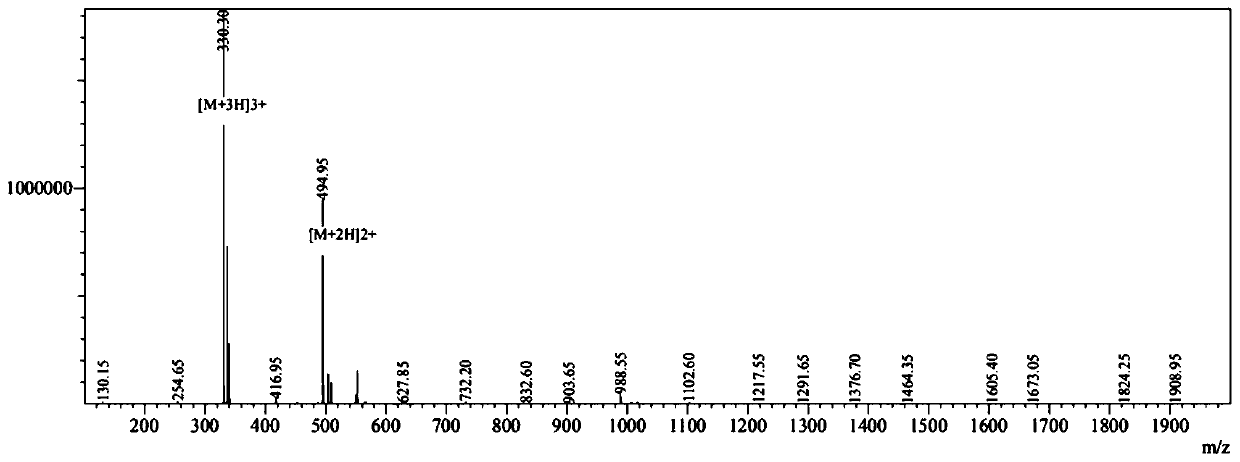
[0066] Example 2 Antimicrobial peptide CPeptide-B: R-R-W-W-R-F (Arg-Arg-Trp-Trp-Arg-Phe) Synthesis Reference Example 1, wherein the (4) step amino acid coupling is (Fmoc-Trp(Pbf)-OH, Fmoc-Trp(Pbf)-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Phe(Pbf)-OH).
[0067] The prepared antimicrobial peptide CPeptide-B was analyzed by mass spectrometry, and the molecular weights shown in the mass spectrum were 987.90, and the theoretical value calculated from the peptide sequence was 988.167, which proved that the prepared peptide was the designed CPeptide-B.
Embodiment 3
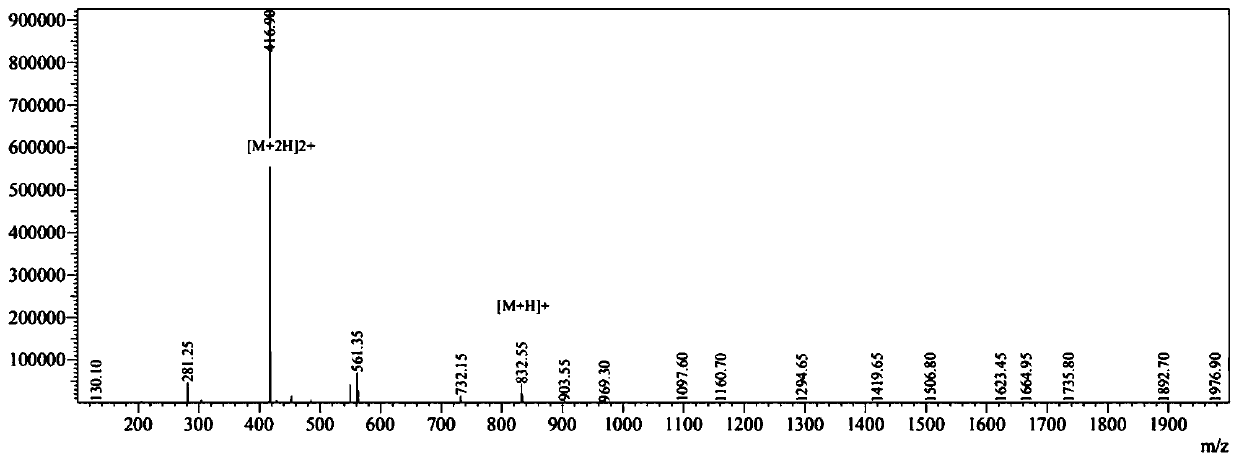
[0068] Embodiment 3 antimicrobial peptide CPeptide-C:R-W-W-R-F (Arg-Trp-Trp-Arg-Phe) synthesis
[0069] With reference to Example 1, wherein (3) step coupling amino acid is Fmoc-Trp(Pbf)-OH, (4) coupling amino acid is successively (Fmoc-Trp(Pbf)-OH, Fmoc-Arg(Pbf)-OH, OH, Fmoc-Phe(Pbf)-OH).
[0070] The prepared antimicrobial peptide CPeptide-C was analyzed by mass spectrometry, and the molecular weights shown in the mass spectrometry were 831.80, and the theoretical value calculated from the polypeptide sequence was 831.979. It is proved that the prepared polypeptide is the designed CPeptide-C antimicrobial peptide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com