Preparation method of near-infrared light-controlled visual medicine carrier
A near-infrared light and drug technology, applied in drug delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] This general formula Im, the synthetic method of n-P-DTPA comprises the following steps:
[0070] (1) Dehydration reaction: Add diethylenetriaminepentaacetic acid, anhydrous acetic anhydride and anhydrous pyridine into a single-necked flask, under the protection of nitrogen, heat to reflux, react for 12-24 hours, suction filter, and wash the filter cake to no color, and vacuum-dried at 50-80°C to obtain diethylenetriaminepentaacetic dianhydride.
[0071]
[0072] The synthesis of formula 1 diethylenetriamine bisanhydride (DTPAA)
[0073] (2) Hydroxyl substitution reaction: 4-bromomethyl-3-nitrobenzoic acid and potassium carbonate were dissolved in a mixed solution of acetone and water, refluxed for 5 hours, the reaction solution was adjusted to acidity, extracted three times with ethyl acetate, The organic layers were combined, dried over anhydrous sodium sulfate, and the spin-dried tan 4-hydroxymethyl-3-nitrobenzoic acid was filtered.
[0074]
[0075] Synthesi...
Embodiment 1
[0101] Example 1 14,14-P-DTPA and biotin-modified upconversion nanoparticles NaYF 4 ,Yb 0.2 / Tm 0.005 compound
[0102] (1) Synthesis of oil-soluble upconversion nanoparticles NaYF 4 ,Yb 0.2 / Tm 0.005 (OA-UCNPs)
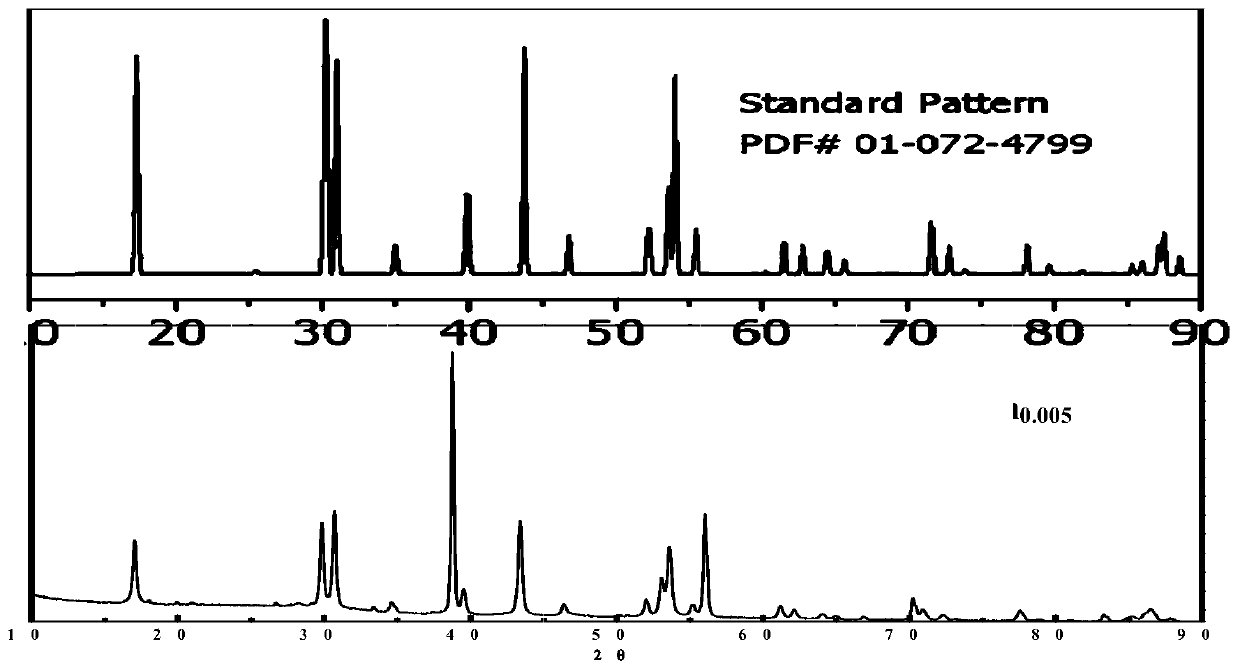
[0103] 272mg CF 3 COONa(2mmol),394.3mg Y(CF 3 COO) 3 (0.795mmol), 102.4mg Yb (CF 3 COO) 3 (0.2mmol) and 2.54mg Tm (CF 3 COO) 3 (0.005mmol) was dissolved in 10mL oleic acid and 10mL octadecene and placed in a three-necked flask, heated in vacuum at 120°C to remove water for 1h, then rapidly raised to 320°C, and reacted for 1h. Naturally cooled to room temperature, methanol was added to wash and centrifuge to obtain a white solid. The white solid is dried, and its XRD diffraction pattern is measured by an X-ray diffractometer and compared with a standard card such as figure 1 , indicating that the structure is correct.
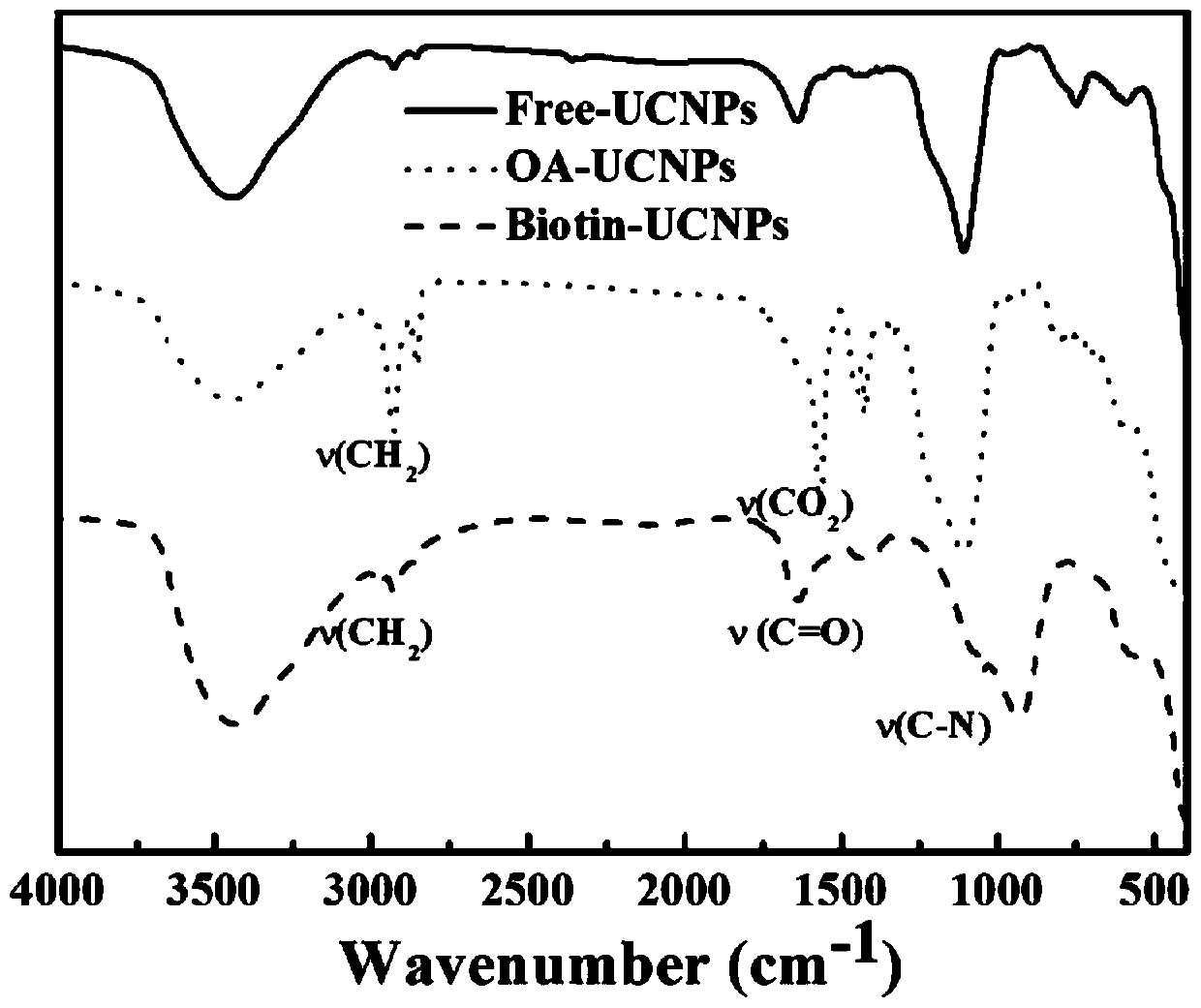
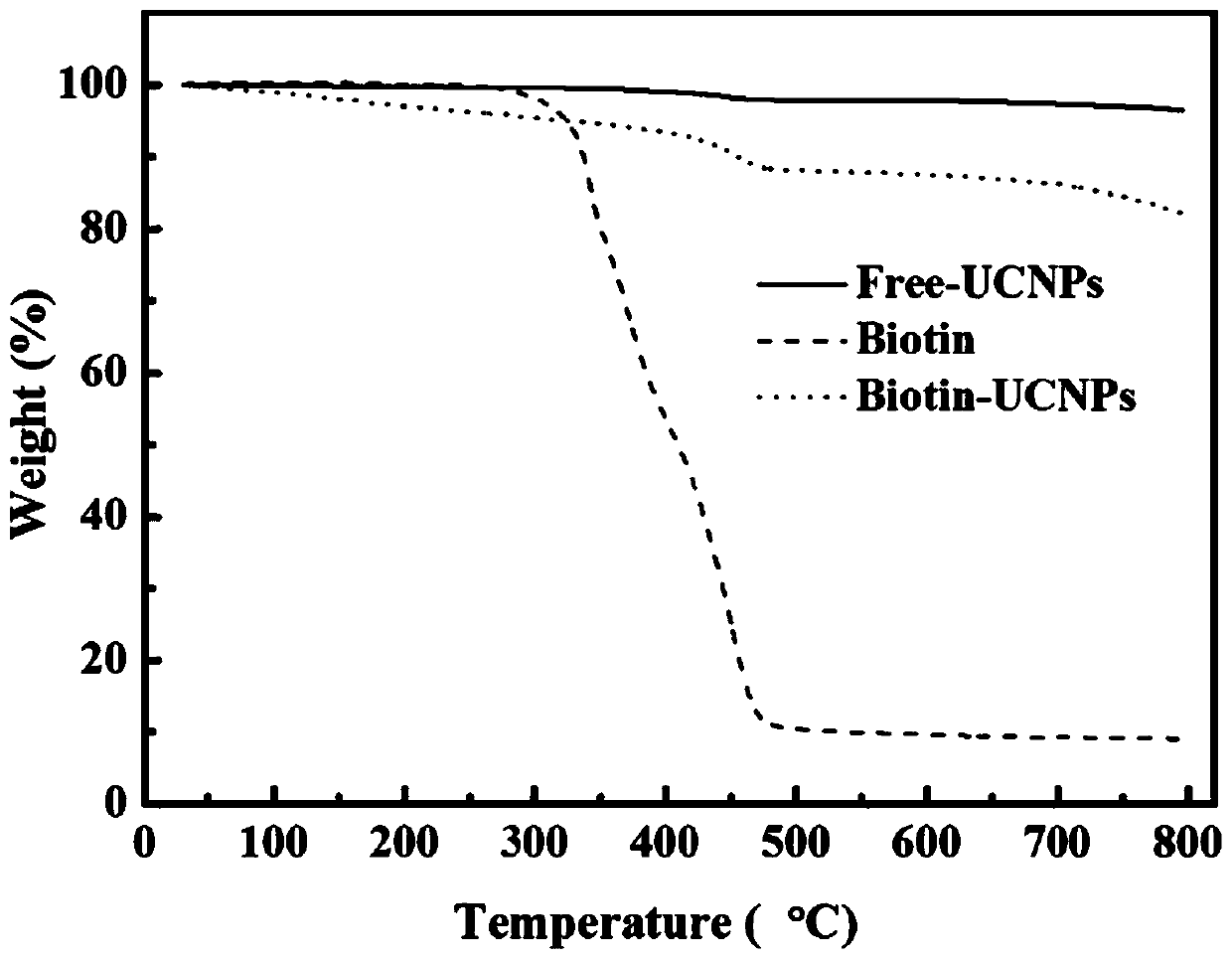
[0104] (2) Synthesis of biotin-modified upconversion nanoparticles NaYF 4 ,Yb 0.2 / Tm 0.005 (Biotin-UCNPs)
[0105] The resulting s...
Embodiment 2
[0116] Example 2 10,10-P-DTPA and 2-aminoethylphosphonic acid modified up-conversion nanoparticles NaYF 4 ,Yb 0.2 / Tm 0.005 compound
[0117] (1) Synthesis of oil-soluble upconversion nanoparticles NaYF 4 ,Yb 0.2 / Tm 0.005 (OA-UCNPs)
[0118] As in Example 1(1)
[0119] (2) Synthesis of 2-aminoethylphosphonic acid modified upconversion nanoparticles NaYF 4 ,Yb 0.2 / Tm 0.005 (AEP-UCNPs)
[0120] Add 5 mL of 0.1M hydrochloric acid solution to the obtained solid and sonicate for 2 h, extract the washed oleic acid with ether, and centrifuge to obtain a white solid. Vigorously stir the resulting white solid and 5mL 2-aminoethylphosphonic acid solution for 2h, add the resulting dispersed solution into 10mL ethylene glycol, stir at 140°C for 1h to remove water, and transfer it to a 20mL autoclave, 130 °C for 3 h, centrifuged to obtain a white solid, which was dispersed in aqueous solution.
[0121] (3) 10,10-P-DTPA encapsulated 2-aminoethylphosphonic acid modified upconve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com