A fluorescent probe for detecting glutathione and its preparation method and application
A fluorescent probe and glutathione technology, applied in the field of analysis and detection, can solve the problems of difficult to achieve accurate glutathione analysis, fluorescent probe interference, etc., and achieve easy application and promotion, intuitive results, and strong anti-interference. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
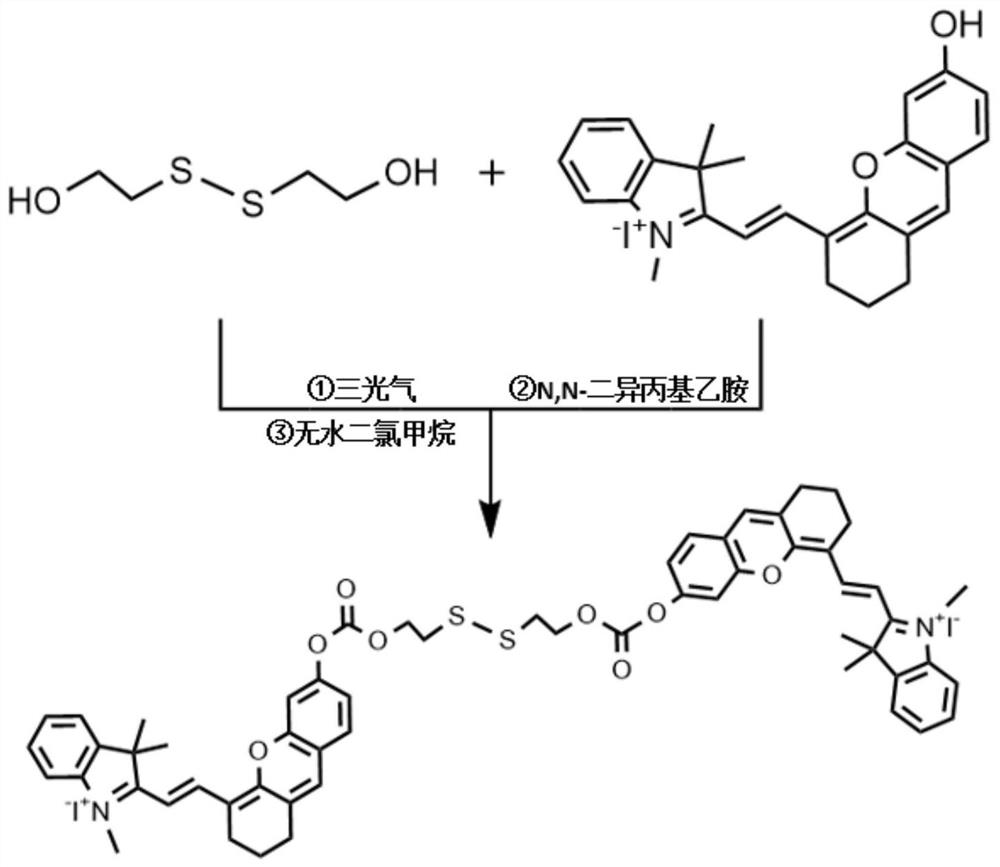
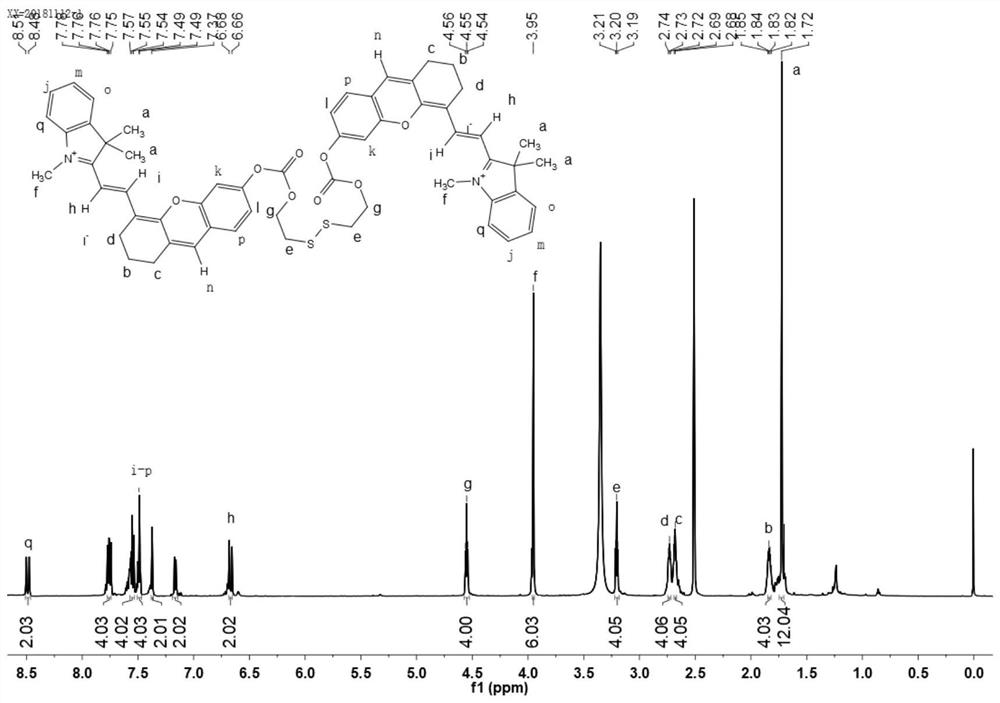
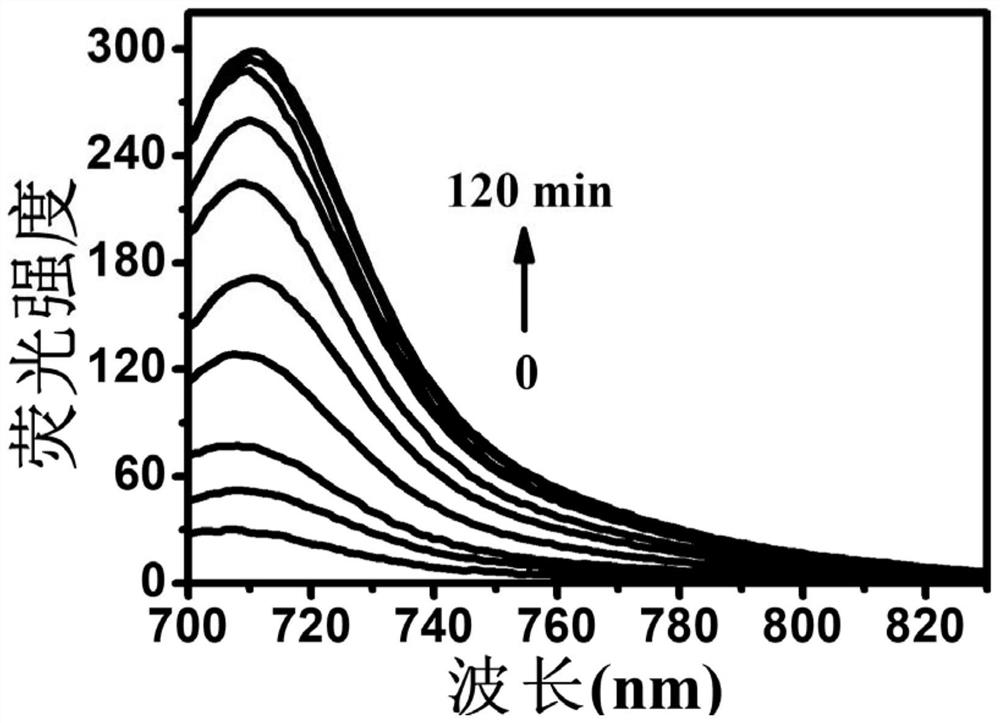
[0046] Dissolve 0.077g 2-hydroxyethyl disulfide in anhydrous dichloromethane, under the protection of inert gas, dissolve 0.444g triphosgene and 400μL N,N-diisopropylethylamine in anhydrous dichloromethane After methane was slowly added dropwise, the reaction was first performed at a low temperature of 0°C for 20 minutes, then at room temperature for 4 hours, and then dried under reduced pressure to obtain a reaction intermediate product. Mix 250 μL of N,N-diisopropylethylamine and 0.537 g of (E)-2-(2-(6-hydroxy-2,3-dihydro-1H-anthracen-4-yl)vinyl)-1, 3,3-trimethyl-3H-indole-1-iodide was dissolved in anhydrous dichloromethane, and under the protection of an inert gas, the above reaction intermediate was dissolved in anhydrous dichloromethane and slowly added dropwise to In the solution, react at room temperature for 24 hours, remove the organic solvent by rotary evaporation, and purify by column chromatography (dichloromethane: ethyl acetate: methanol, V / V / V=8:4:1) to obtain g...
Embodiment 2
[0049] Dissolve 0.077g 2-hydroxyethyl disulfide in anhydrous dichloromethane, under the protection of inert gas, dissolve 0.474g triphosgene and 450μL N,N-diisopropylethylamine in anhydrous dichloromethane After methane was slowly added dropwise, the reaction was first performed at a low temperature of 10°C for 15 minutes, then at room temperature for 6 hours, and then dried under reduced pressure to obtain a reaction intermediate product. Mix 300 μL of N,N-diisopropylethylamine and 0.511 g of (E)-2-(2-(6-hydroxy-2,3-dihydro-1H-anthracen-4-yl)vinyl)-1, 3,3-trimethyl-3H-indole-1-iodide was dissolved in anhydrous dichloromethane, and under the protection of an inert gas, the above reaction intermediate was dissolved in anhydrous dichloromethane and slowly added dropwise to In the solution, react at room temperature for 18 hours, remove the organic solvent by rotary evaporation, and purify by column chromatography (dichloromethane: ethyl acetate: methanol, V / V / V=8:4:1) to obtain ...
Embodiment 3
[0052] Dissolve 0.077g 2-hydroxyethyl disulfide in anhydrous dichloromethane, under the protection of inert gas, dissolve 0.459g triphosgene and 500μL N,N-diisopropylethylamine in anhydrous dichloromethane After methane was slowly added dropwise, the reaction was performed at a low temperature of 5°C for 25 minutes, and then at room temperature for 5 hours, and then dried under reduced pressure to obtain a reaction intermediate product. Mix 200 μL of N,N-diisopropylethylamine and 0.524 g of (E)-2-(2-(6-hydroxy-2,3-dihydro-1H-anthracen-4-yl)ethenyl)-1, 3,3-trimethyl-3H-indole-1-iodide was dissolved in anhydrous dichloromethane, and under the protection of an inert gas, the above reaction intermediate was dissolved in anhydrous dichloromethane and slowly added dropwise to In the solution, react at room temperature for 20 hours, remove the organic solvent by rotary evaporation, and purify by column chromatography (dichloromethane: ethyl acetate: methanol, V / V / V=8:4:1) to obtain g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com