Silicon-based modified isonitrile metal salt catalyst and preparation method and application thereof
A catalyst and metal salt technology, which is applied in the field of isocyanide metal salt catalyst and its preparation, can solve the problems of poor compatibility between the catalyst and the reaction substrate, unsatisfactory yield of the target product, and low catalytic activity, and achieve the goal of improving The effect of product yield, inhibition of by-product formation, wide substrate universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
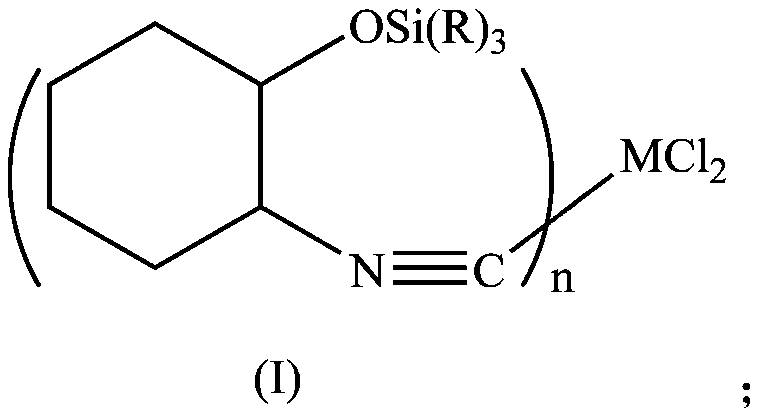
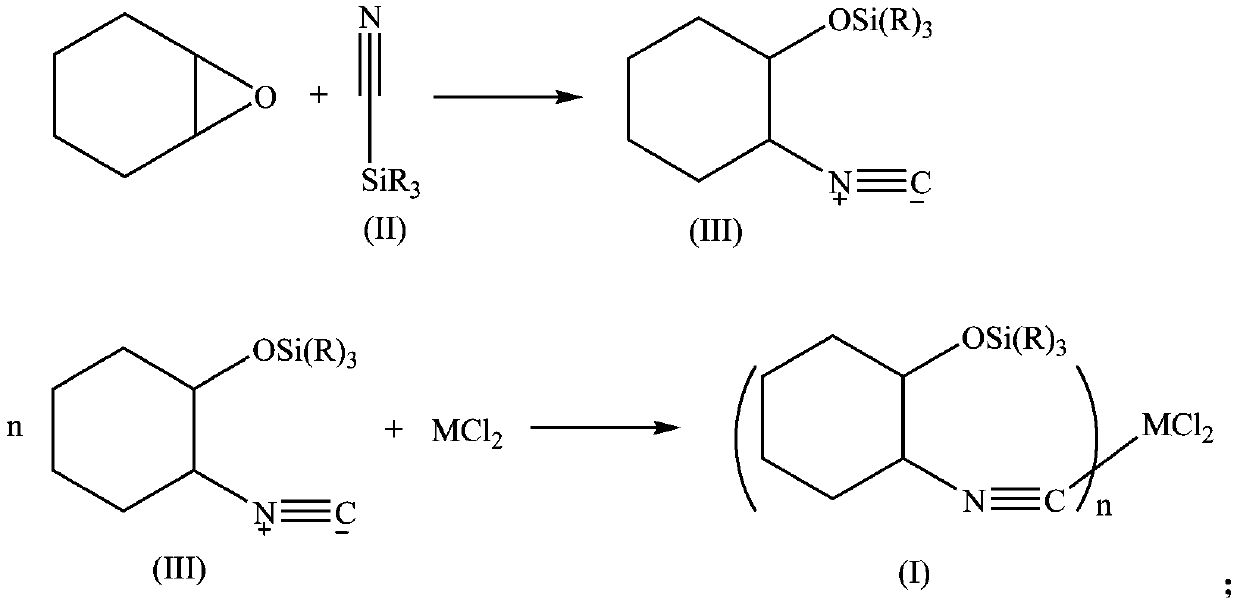
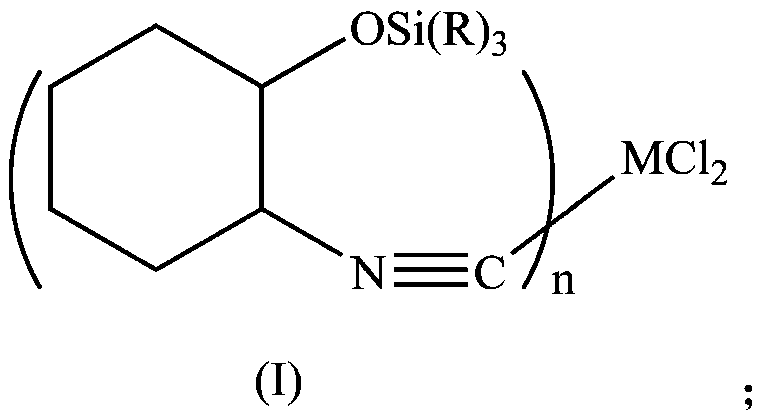
[0032] In a 250mL three-necked flask, add 150mL of dichloromethane, cyclohexene oxide (10.1g, 0.1mol), trimethylsilylcyanide (19.8g, 0.2mol), zinc iodide (0.3g, 0.001mol), and stir to mix. Heat to reflux for 2h, cool, filter with suction, spin dry the solvent, and dry in vacuo to obtain a solid product.
[0033] Dissolve the obtained solid product with 100 mL of FeCl 2 4H 2 O (9.94g, 0.05mol) of tetrahydrofuran was mixed, stirred overnight, suction filtered, washed with deionized water, washed with acetone, and dried in vacuo to obtain 22g of solid product Fe[CNC 6 h 12 Si(CH 3 ) 3 ] 2 Cl 2 .
Embodiment 2
[0035] In a 250mL three-necked flask, add 150mL of dichloromethane, cyclohexene oxide (10.1g, 0.1mol), trimethoxysilylcyanide (19.8g, 0.2mol), zinc iodide (0.3g, 0.001mol), and stir to mix. Heat to reflux for 2h, cool, filter with suction, spin dry the solvent, and dry in vacuo to obtain a solid product.
[0036] Dissolve the obtained solid product with 100 mL of FeCl 2 4H 2 O (9.94g, 0.05mol) of tetrahydrofuran was mixed, stirred overnight, suction filtered, washed with deionized water, washed with acetone, and dried in vacuo to obtain 20g of solid product Fe[CNC 6 h 12 Si(OCH 3 ) 3 ] 2 Cl 2 .
Embodiment 3
[0038] In a 250mL three-necked flask, add 150mL of dichloromethane, cyclohexene oxide (10.1g, 0.1mol), triethylsilylcyanide (19.8g, 0.2mol), zinc iodide (0.3g, 0.001mol), stir and heat Reflux for 2h, cool, filter with suction, spin dry the solvent, and dry in vacuo to obtain a solid product.
[0039] Dissolve the obtained solid product with 100 mL of FeCl 2 4H 2 O (9.94g, 0.05mol) of tetrahydrofuran was mixed, stirred overnight, suction filtered, washed with deionized water, washed with acetone, and dried in vacuo to obtain 26g of solid product Fe[CNC 6 h 12 Si(CH 2 CH 3 ) 3 ] 2 Cl 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com