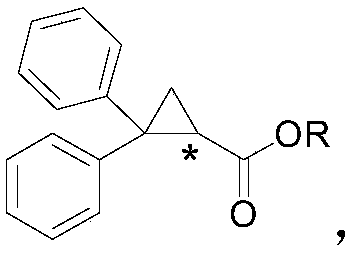
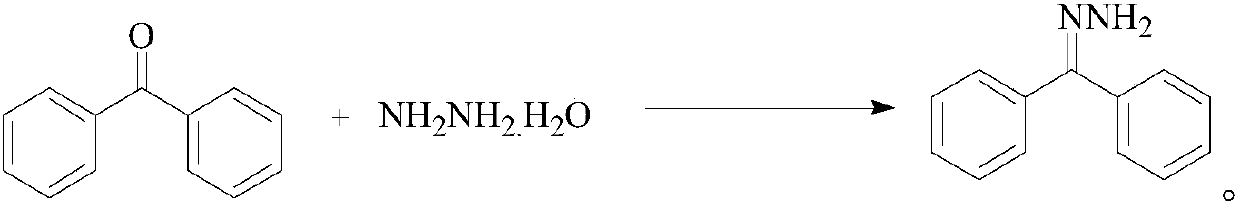2,2-diphenylcyclopropyl compound and synthesis method thereof
A technology of diphenylcyclopropanecarboxylic acid and synthesis method, applied in the directions of organic chemistry method, chemical instrument and method, preparation of organic compounds, etc., can solve the problem of unfavorable industrial production of cyclopropane compounds, long reaction time, low yield, etc. It is easy to industrialize large-scale production, the method is simple, and the synthesis method is simple.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The present embodiment provides a kind of methyl 2,2-diphenylcyclopropanecarboxylate, its structural formula is:
[0027]
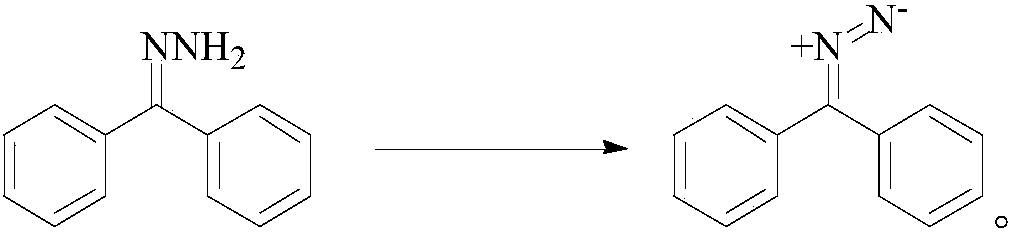
[0028] The synthetic method of this compound comprises: get 3.64g benzophenone and 10ml 50% hydrazine hydrate in 100ml ethyl acetate at 40 ℃ reflux 2.5h, cool crystallization after reaction finishes and obtain benzophenone hydrazone; Add 1.82g of manganese dioxide to the anhydrous ethyl acetate solution of hydrazone, stir at 30°C for 1.2h, distill off the ethyl acetate solvent under reduced pressure at room temperature after the reaction, and then redissolve the residue In ethyl acetate, the ethyl acetate solvent was distilled off again under reduced pressure to obtain diphenyldiazomethane; diphenyldiazomethane was reacted with 1.8g methyl acrylate in ethyl acetate solvent at 50°C for 1.5 h, after the reaction was over, the ethyl acetate solvent was distilled off under reduced pressure at room temperature, and then recrystallized with 95% ethano...
Embodiment 2
[0030] The present embodiment provides a kind of chloromethyl 2,2-diphenylcyclopropanecarboxylate, and its structural formula is:
[0031]
[0032] Get 3.64g of benzophenone and 10ml of 50% hydrazine hydrate in 100ml of ethyl acetate solvent at 70 ℃ reflux 2.5h, cooling crystallization after the end of the reaction to obtain benzophenone hydrazone; Add 1.82g of manganese dioxide to the ethyl ester solution, stir at 60°C for 1.2h, and distill off the ethyl acetate solvent under reduced pressure at room temperature after the reaction, and then redissolve the residue in ethyl acetate , and distilled off the ethyl acetate solvent again under reduced pressure to obtain diphenyldiazomethane; react diphenyldiazomethane with 2.4g chloromethyl acrylate in ethyl acetate solvent at 40°C for 1.5h, and the reaction ends After that, the ethyl acetate solvent was distilled off under reduced pressure at room temperature, and then recrystallized with 95% ethanol to obtain 5.53 g of the targ...
Embodiment 3
[0034] This embodiment provides a kind of ethyl 2,2-diphenylcyclopropanecarboxylate, its structural formula is:
[0035]
[0036] The synthetic method of this compound comprises: get 3.64g benzophenone and 5ml 70% hydrazine hydrate in 100ml ethyl acetate at 80 ℃ reflux 3h, cooling crystallization after reaction finishes and obtains benzophenone hydrazone; To benzophenone hydrazone Add 1.74g of manganese dioxide to the anhydrous ethyl acetate solution, and stir at 40°C for 1.5h. After the reaction is over, the ethyl acetate solvent is distilled off under reduced pressure at room temperature, and then the residue is redissolved in In ethyl acetate, the ethyl acetate solvent was distilled off under reduced pressure again to obtain diphenyldiazomethane; diphenyldiazomethane was reacted with 2.23g ethyl acrylate in ethyl acetate solvent at 65°C for 1.5h After the reaction, the ethyl acetate solvent was distilled off under reduced pressure at room temperature, and then recrystall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com