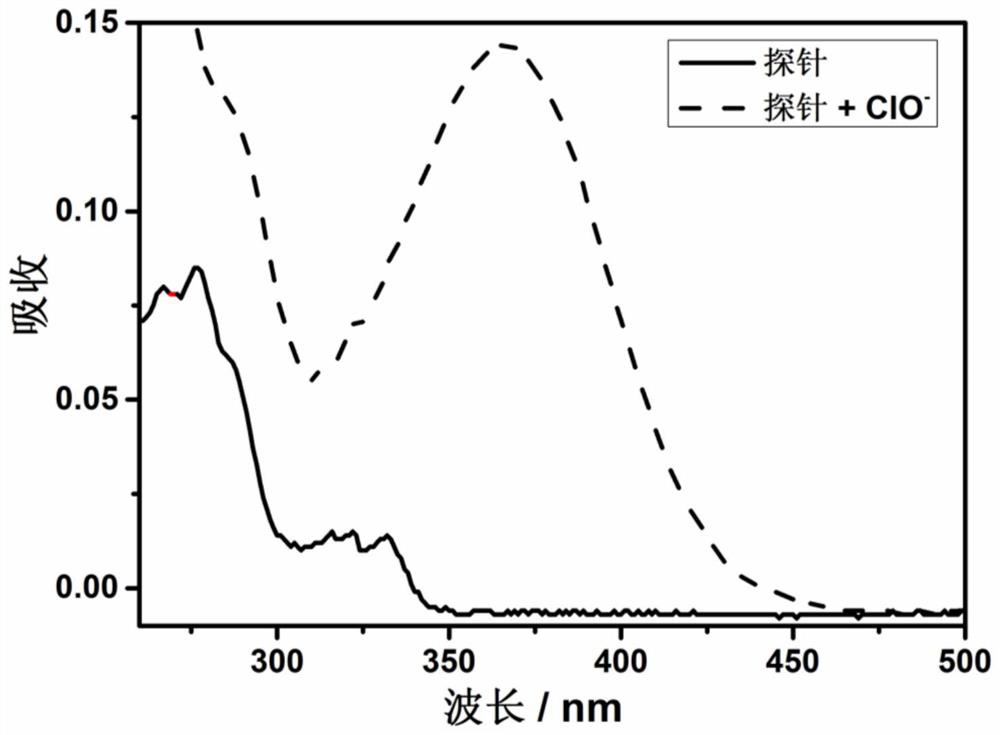
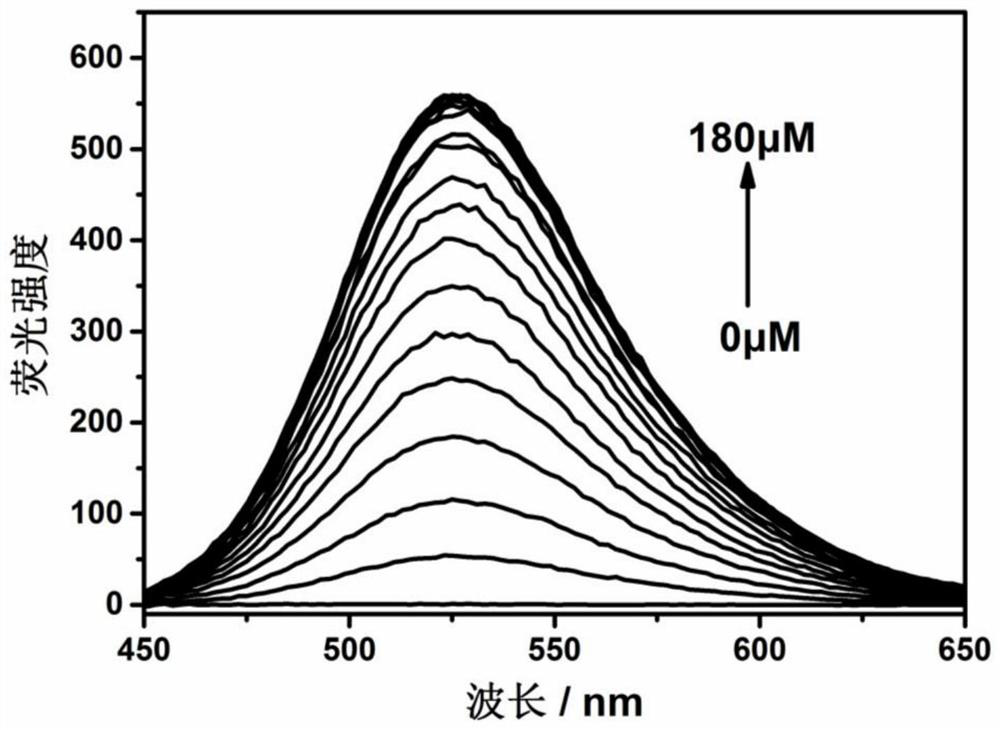
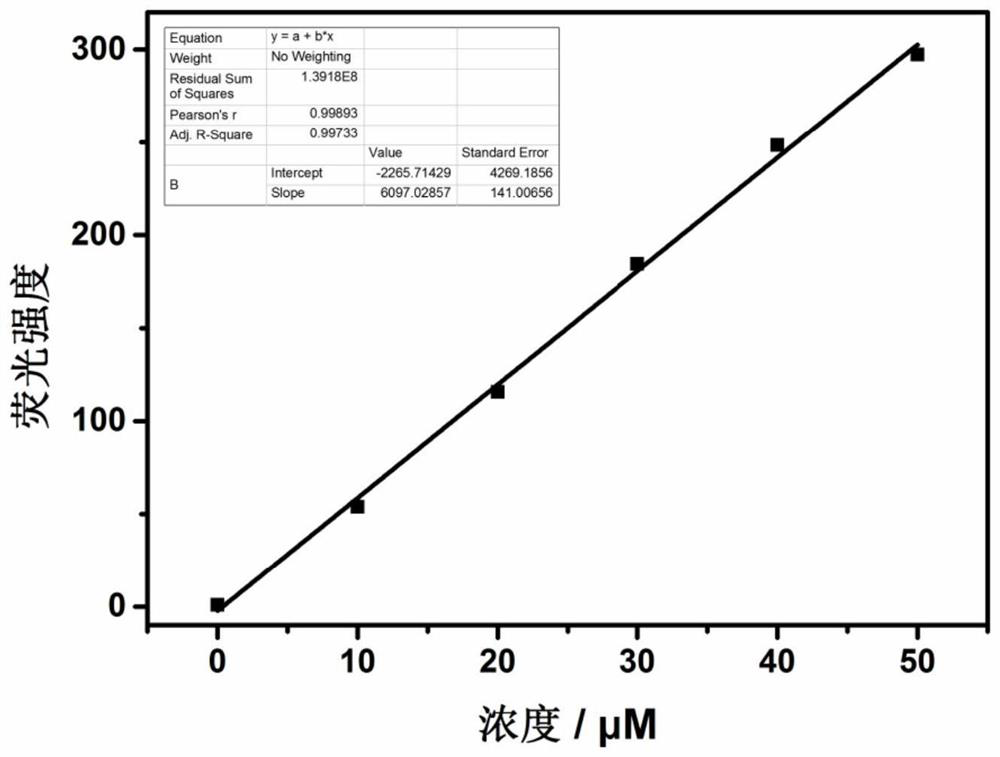
Fluorescent probe for detecting hypochlorite ion, preparation method and application thereof
A hypochlorite and fluorescent probe technology, applied in the field of fluorescent probes, can solve the problems of affecting the sensitivity and selectivity of the sensing method, unable to monitor the fluctuation of hypochlorous acid in real time, unstable basic level, etc. High rate, low biotoxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 detects the preparation of the fluorescent probe of hypochlorite ion
[0036] Under argon protection, 172.18 mg (1 mmol) of 6-hydroxy-2-naphthaldehyde was dissolved in 10 ml of dichloromethane, and 104 μl (1.5 mmol) of dimercaptoethanol and 18 μl (0.1 mmol) of TMSOTf (trifluoromethanesulfonate methyl silicon ester) at room temperature 25°C for 30min; after the reaction was completed, the reaction solution was washed with saturated brine and dried over anhydrous sodium sulfate; after drying, the solvent was spin-dried, and purified by silica gel column chromatography (eluent was ethyl acetate Esters: petroleum ether = 1:4 (V: V)), to obtain 135 mg of compound 6-(1,3-oxathiolan-2-yl)naphthalene-2-ol (60% yield), namely A fluorescent probe for detecting hypochlorite ions has a synthetic route as follows:
[0037]
[0038] The H NMR spectrum data of the probe molecules are as follows:
[0039] probe- 1 H NMR (400MHz, DMSO): δ9.77(s, 1H), 7.80(s, 1H), 7.76...
Embodiment 2
[0040] Embodiment 2 detects the preparation of the fluorescent probe of hypochlorite ion
[0041] Under argon protection, 344.36 mg (2 mmol) of 6-hydroxy-2-naphthaldehyde was dissolved in 10 ml of dichloromethane, and 208 μl (3 mmol) of dimercaptoethanol and 36 μl (0.2 mmol) of TMSOTf (trimethyl trifluoromethanesulfonate) were added base silicon ester) at 20°C for 20 minutes; after the reaction was completed, the reaction solution was washed with saturated brine and dried over anhydrous sodium sulfate; after drying, the solvent was spin-dried, and purified by silica gel column chromatography (eluent: ethyl acetate: Petroleum ether=1:4 (V:V)), to obtain 261 mg of compound 6-(1,3-oxathiolan-2-yl)naphthalene-2-ol (58% yield), that is, the detection times Fluorescent probe for chlorate ion.
Embodiment 3
[0042] Embodiment 3 detects the preparation of the fluorescent probe of hypochlorite ion
[0043] Under argon protection, 258.57 mg (1.5 mmol) of 6-hydroxy-2-naphthaldehyde was dissolved in 10 ml of dichloromethane, and 156 μl (2.25 mmol) of dimercaptoethanol and 27 μl (0.15 mmol) of TMSOTf (trifluoromethanesulfonic acid Trimethylsilyl ester) was reacted at 40°C for 10 min; after the reaction was completed, the reaction solution was washed successively with saturated brine, and dried over anhydrous sodium sulfate; after drying, the solvent was spin-dried, and then purified by silica gel column chromatography (eluent was ethyl acetate Esters: Petroleum ether = 1:4 (V:V)), to obtain 205.9 mg of compound 6-(1,3-oxathiolan-2-yl)naphthalene-2-ol (61% yield), namely A fluorescent probe for the detection of hypochlorite ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com