Use of glycine in aspect of preparation of drug delivery reinforcer and cell transplantation reagent
A technology for enhanced delivery and preparation of drugs, applied in the field of biomedicine, can solve the problems of ineffective effect and low transplantation rate in aging muscle atrophy mice
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
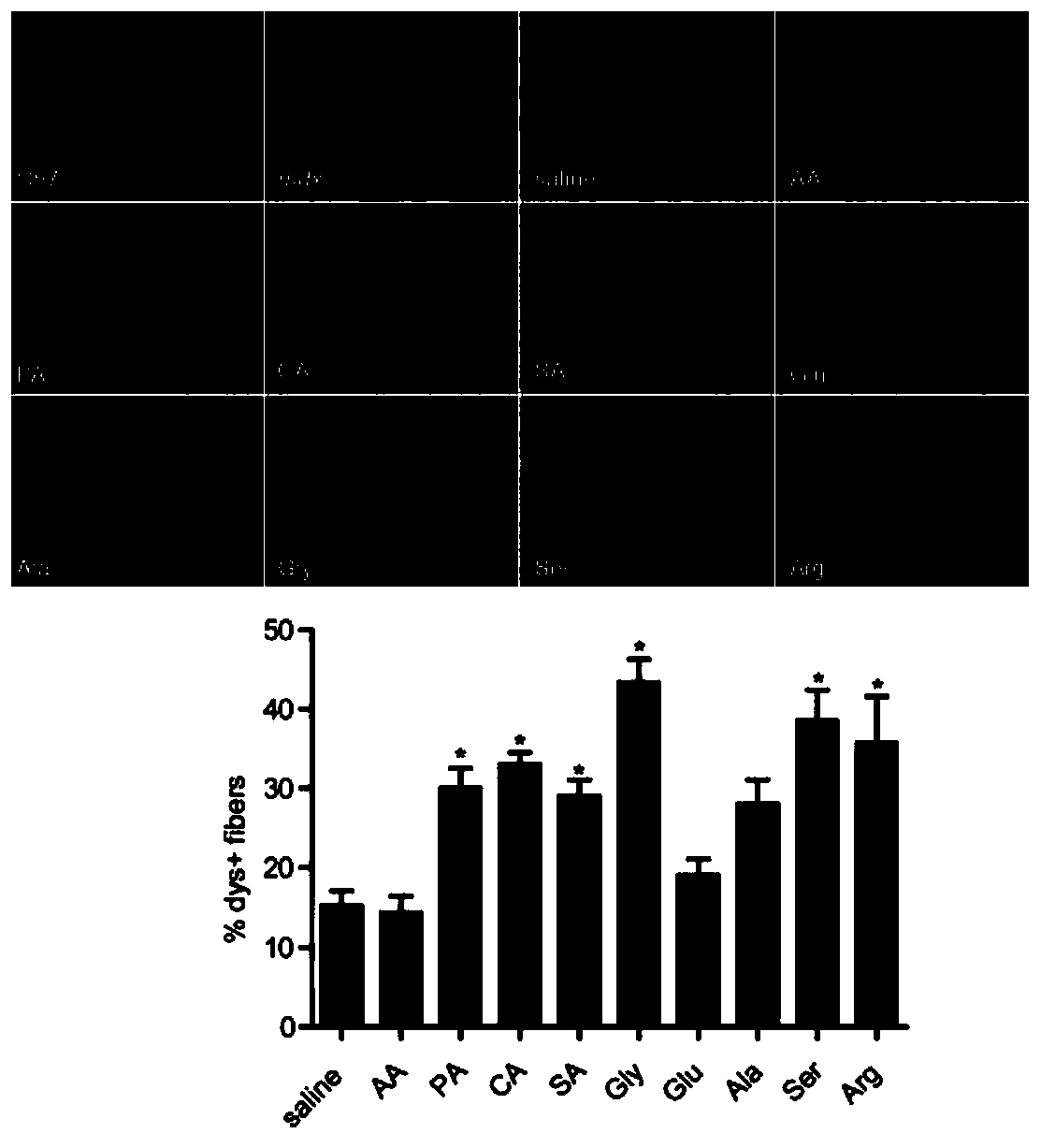
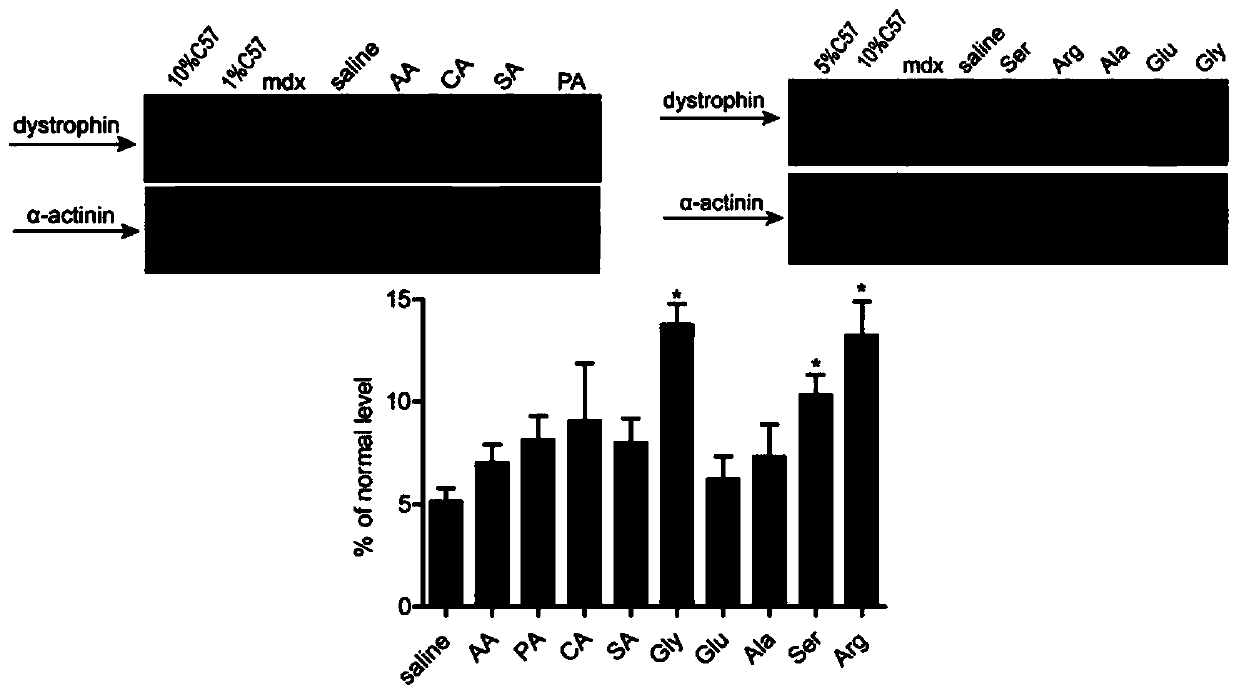
[0129] Different small molecular compounds were mixed with PMO, the total volume was 40 μl, the final concentration of small molecular compounds was 5% (mass volume ratio), and the dose of PMO was 2 μg. Injected into the tibialis anterior muscle, the control group was a mixture of normal saline and PMO, administered once to adult mdx mice at 6-8 weeks, and samples were taken for detection two weeks later. Immunohistochemistry (IHC) and western-blot were used to detect the expression level of dystrophin protein. figure 1 It is the IHC result, and the red part is the dystrophin protein. From the IHC results, it can be seen that the positive regions of the dystrophin protein in the glycine group are more than those in other small molecule compound groups. figure 2 It is a western-blot result and a quantitative statistical chart. It can be seen from the results that the expression level of dystrophin protein in the glycine group is higher than that in other groups.
experiment example 2
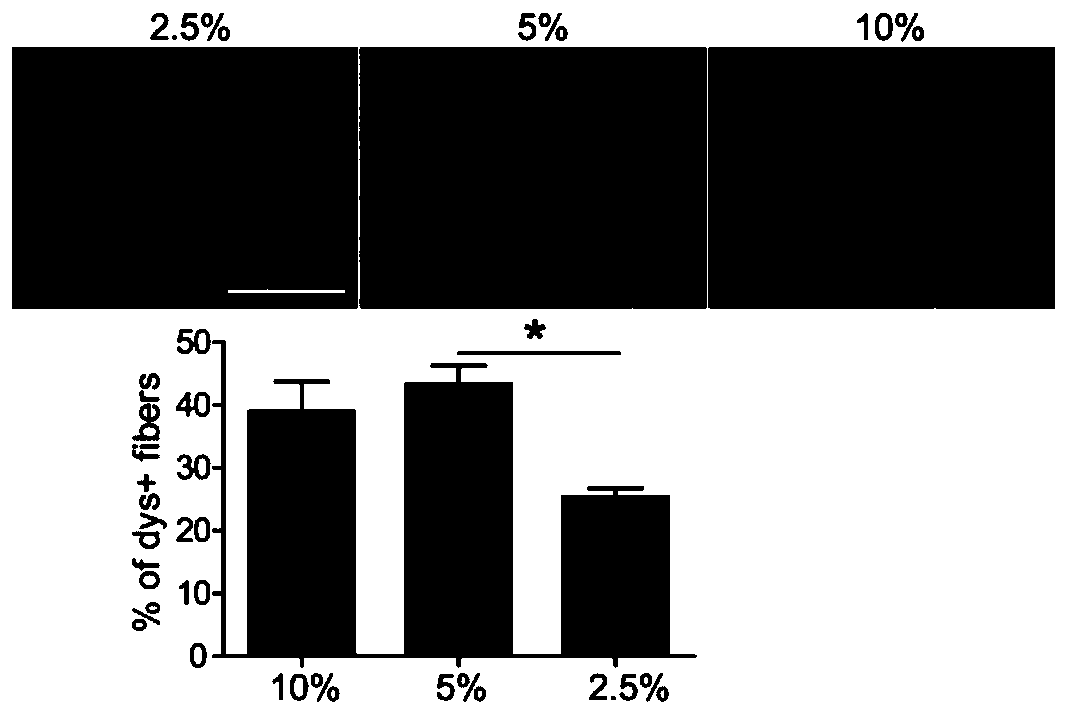
[0131] 2.5%, 5% and 10% glycine were mixed with 2 μg PMO respectively, and the total volume was 40 μl, wherein, the final concentration of glycine was 2.5%, 5% or 10%, respectively, and were injected locally once in the tibialis anterior muscle of mdx mice, Samples were taken after two weeks. Immunohistochemistry (IHC) was used to detect the expression level of dystrophin protein. image 3 IHC staining results and quantitative statistical results, the red part is dystrophin protein, from the IHC results, it can be seen that the 5% glycine group has more dystrophin protein positive areas in the nucleic acid drug mixture than 2.5%; similar to the 10% glycine group. Figure 4 It is the result of western blot and quantitative statistics. The quantitative analysis results also show that glycine improves the drug activity of PMO, and the 5% glycine group is better than the 2.5% group, and the effect is close to that of the 10% glycine group.
experiment example 3
[0133] Mix glycine with 2'Ome RNA, B-MSP-PMO, PNA and Pip5e-PMO respectively in a total volume of 40 μl, wherein the final concentration of glycine is 5%, containing 5 μg of 2'Ome or 1 μg of B-MSP-PMO or PNA 5 μg or Pip5e-PMO 1 μg were injected locally in the tibialis anterior muscle. In the control group, normal saline was mixed with respective nucleic acid drugs, and the others were the same as the glycine group, only glycine was replaced with normal saline. They were injected into the tibialis anterior muscle of mdx mice once, and samples were taken two weeks later. Immunohistochemistry (IHC) was used to detect the expression level of dystrophin protein. Figure 5 It is the result of IHC staining and quantitative statistics. The red part is the dystrophin protein. From the IHC results, it can be seen that the glycine group has more dystrophin protein positive areas in different nucleic acid drug mixtures than the control group. Figure 6 It is the result of western blot a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com