Asymmetric 9,10-dithienylanthracene fluorescence compound, and preparation method and application thereof
A bisthienyl anthracene and fluorescent compound technology, applied in chemical instruments and methods, fluorescence/phosphorescence, hydroxyanthraquinone dyes, etc., can solve the problem of rare AIE compound molecules, and achieve simple preparation methods, low preparation costs, and high preparation conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
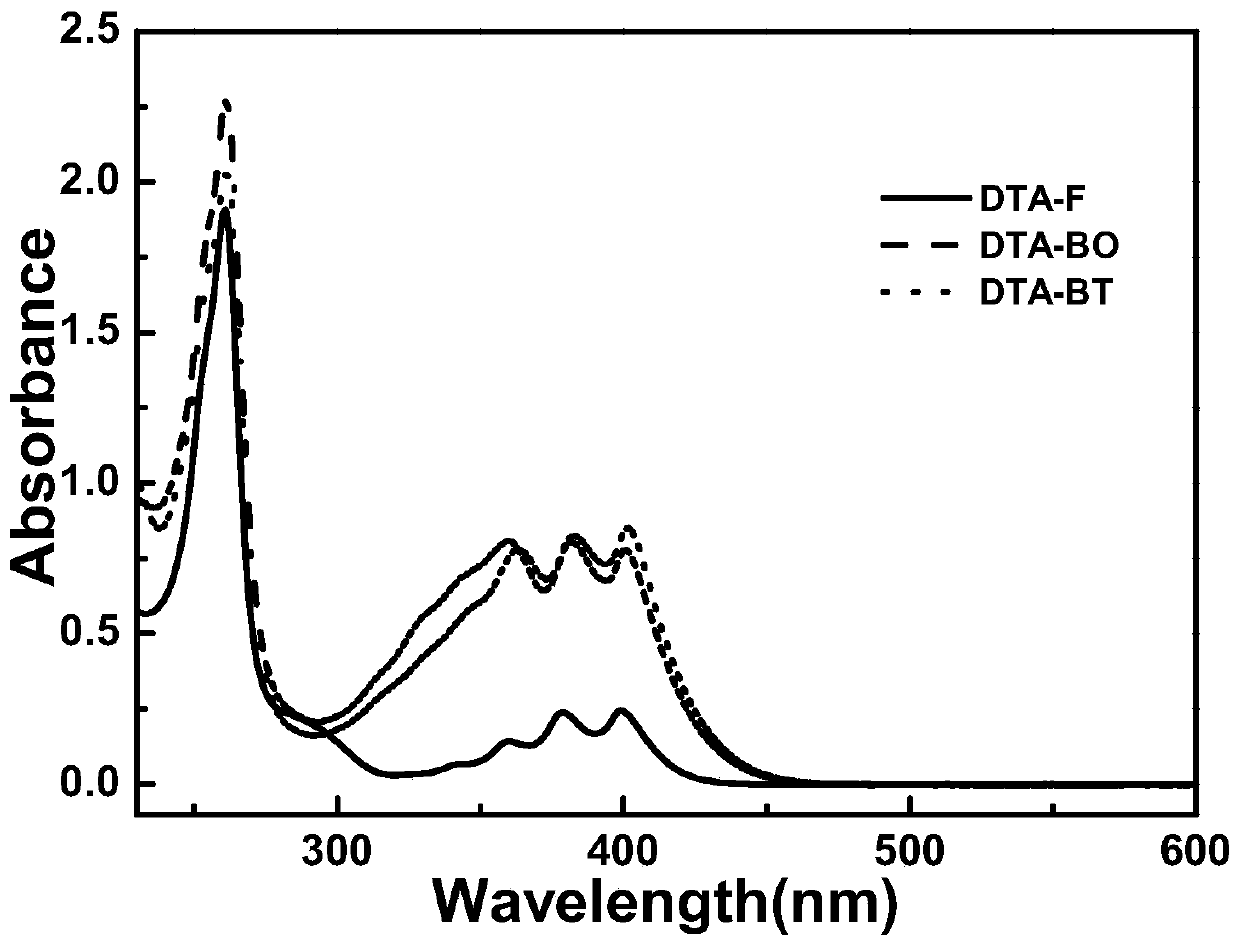
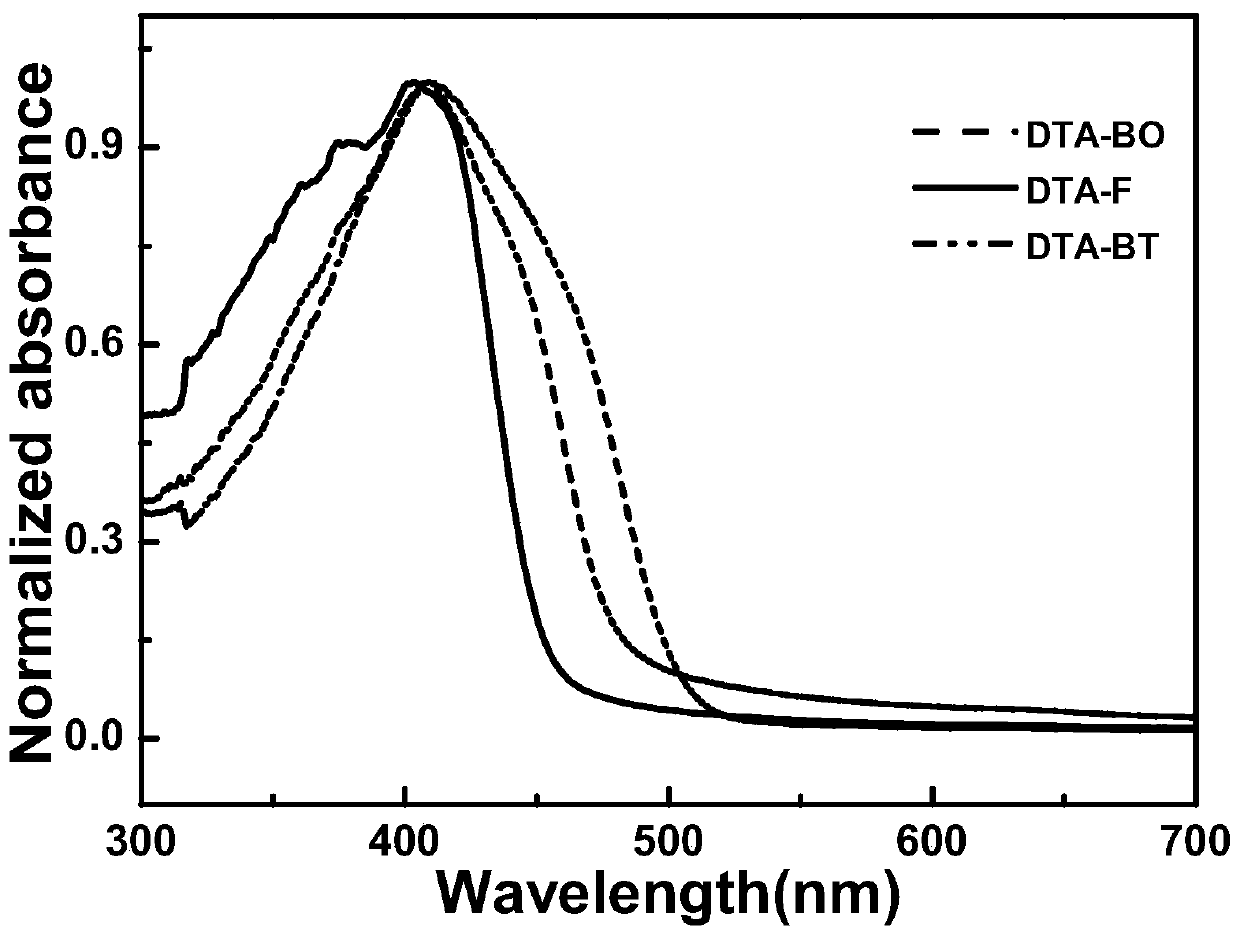
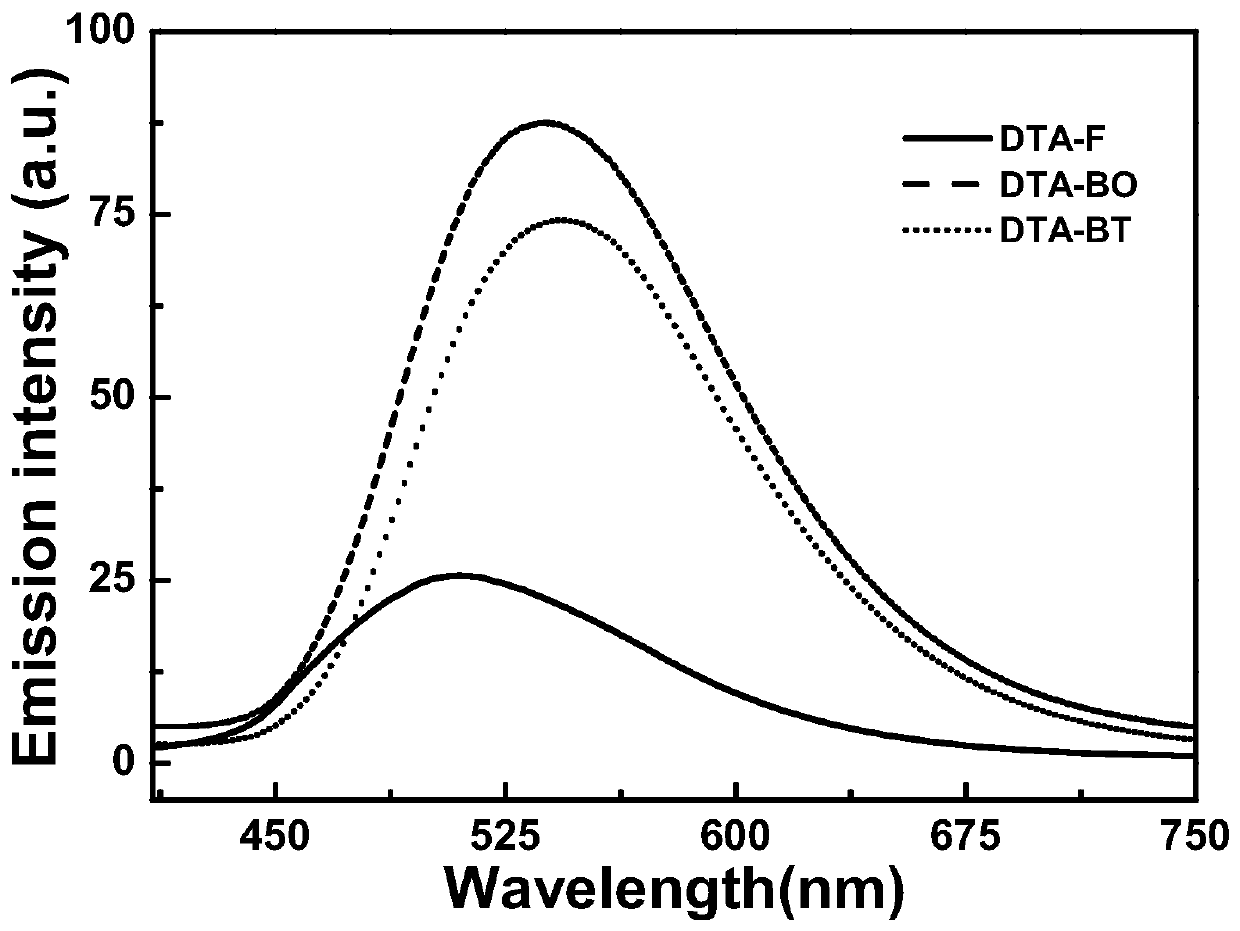
Image
Examples
preparation example Construction
[0057] The present invention provides a preparation method for the asymmetric 9,10-bisthienylanthracene fluorescent compound described in the above scheme, comprising the following steps:
[0058] (i) When R in formula I is , the compound shown in formula I is [9-thienyl, 10-(5-formyl-2-thienyl)] anthracene, and the preparation method comprises the following steps:
[0059] (1) Under a protective atmosphere, mix 9,10-dibromoanthracene, thiophene-2-boronic acid, a palladium catalyst, a basic compound and a solvent for a Suzuki coupling reaction to obtain 9-bromo-10-thienylanthracene;
[0060] (2) Under protective atmosphere, 9-bromo-10-thienyl anthracene, 5-formyl-2-thiophene boronic acid, palladium catalyst, basic compound and solvent are mixed for Suzuki coupling reaction to obtain [9-thienyl , 10-(5-formyl-2-thienyl)]anthracene.
[0061] In the present invention, the reaction equation of the Suzuki coupling reaction in the step (1) is as shown in formula V:
[0062]
...
Embodiment 1
[0102] Preparation of [9-thienyl,10-(5-formyl-2-thienyl)]anthracene (DTA-F)
[0103] (1) Preparation of 9-bromo-10-thienyl anthracene:
[0104] Under nitrogen protection, 9,10-dibromoanthracene (1.00g, 3.0mmol) and Pd(PPh 3 ) 4 (0.10g) was dissolved in 80.0mLTHF, after stirring for 30min, thiophene-2-boronic acid (0.46g, 3.6mmol) was added and the concentration was 2.0mol / L Na 2 CO 3 Solution 30.0mL, heated to reflux for 12h, stop the reaction, cooled to room temperature. The solvent was removed by rotary evaporation, extracted with dichloromethane, the organic phases were combined, and dried over anhydrous magnesium sulfate. Suction filtration, the filtrate was rotary evaporated to remove the solvent, and petroleum ether was used as the eluent for column chromatography to obtain a yellow-green solid with a yield of 50%.
[0105] (2) Preparation of [9-thienyl, 10-(5-formyl-2-thienyl)]anthracene (DTA-F):
[0106] Under nitrogen protection, 9-bromo-10-thienylanthracene (1....
Embodiment 2
[0109] Preparation of [9-thienyl, 10-(5-vinylbenzothiazolyl)-2-thienyl]anthracene (DTA-BT):
[0110] Under the protection of nitrogen, potassium tert-butoxide (0.67g, 0.6mmol) and 10.0mL THF were reacted at 0°C for 10min, then 2-methylbenzothiazole (0.07g, 0.45mmol) was dissolved in 8.0mL THF Slowly drop the mixed solution and keep the temperature at 0°C for 1.0h. Then [9-thienyl, 10-(5-formyl-2-thienyl)] anthracene (DTA-F, 0.11g, 0.3mmol) was dissolved in 8.0mL THF, slowly added dropwise, and reacted for 1.0h. After the reaction is complete, add water to terminate the reaction, extract with dichloromethane, combine the organic phases, and dry over anhydrous magnesium sulfate. After suction filtration, the filtrate was rotary evaporated to remove the solvent, and separated by column chromatography using petroleum ether: ethyl acetate = 10:1 as the eluent to obtain a yellow solid with a yield of 50%.
[0111] Structure Identification: 1 H NMR(400MHz,DMSO,ppm):δ7.34(d,1H,thio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com