Synthesis method of eugenol artificial antigen
A technology of artificial antigen and synthesis method, which is applied in chemical instruments and methods, hybrid peptides, animal/human proteins, etc., can solve the problems of high pretreatment requirements, influence on anesthetic residues, consume large manpower and material resources, etc., and achieve the synthesis steps simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
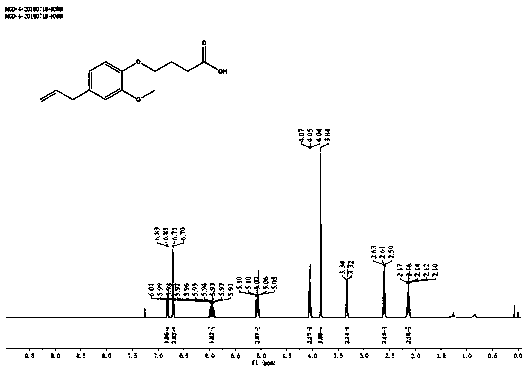
[0027] Example 1 Synthesis of Hapten Euy
[0028]
[0029] MGD_4_1: K 2 CO 3 (378.75 mg, 2.74 mmol) was added to a stirred solution of 4-allyl-2-methoxyphenol (300 mg, 1.83 mmol) in DMF (5 mL). After stirring at room temperature for 15 min, tert-butyl 4-bromobutyrate (428 mg, 1.92 mmol) was slowly added to the mixture. The reaction mixture was then stirred at room temperature for 16 h. Water (50 mL) was added to the reaction mixture, and the mixture was extracted with EtOAc (3 x 25 mL). The combined organic layers were washed with brine (25 mL), and washed with Na 2 SO 4 Drying and concentration in vacuo gave a colorless oil which was purified by flash column chromatography to give tert-butyl 4-(4-allyl-2-methoxyphenoxy)butyrate (462 mg, 1.51 mmol, Yield 82.53%), as a colorless oil, namely the compound MGD_4_1.
[0030] MGD_4: tert-butyl 4-(4-allyl-2-methoxyphenoxy)butyrate (924mg, 3.02mmol) DCM (5mL) solution was cooled and stirred in an ice bath, and TFA was slowly...
Embodiment 2
[0032] Example 2 The preparation method of complete antigen Euy-EDC-BSA is as follows:
[0033] a. Weigh 2.2 mg of the hapten Euy obtained in step (1), 6.7 mg of 1-ethylcarbodiimide hydrochloride, 4.0 mg of N-hydroxysuccinimide, and dissolve in 400 μL N,N-dimethyl Base formamide DMF solution (referred to as liquid A), stirred at room temperature for 4-6h. Take 10mg bovine serum albumin BSA (the molar ratio of Euy to bovine serum albumin BSA is 60︰1), add 2mL pH8.6, 0.01M carbonate buffer solution CB (referred to as B solution), drop A at room temperature solution was slowly added to solution B, and reacted overnight at room temperature; the conjugate Euy-BSA mixture was obtained;
[0034] b. Dialysis: Take a 10cm dialysis bag, boil it in boiling water for 5 minutes, rinse it with 60°C deionized water for 3 minutes, and store it in 4°C deionized water for later use; put the conjugate Euy-EDC-BSA mixture into the dialysis bag, and dialyzed with pH = 7.2, 0.01M phosphate buffer s...
Embodiment 3
[0035] Example 3 Identification of eugenol artificial antigen
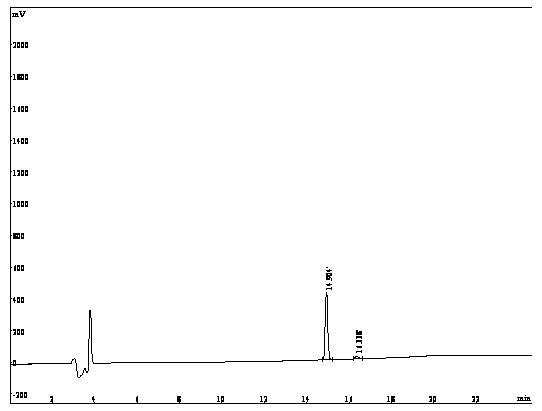
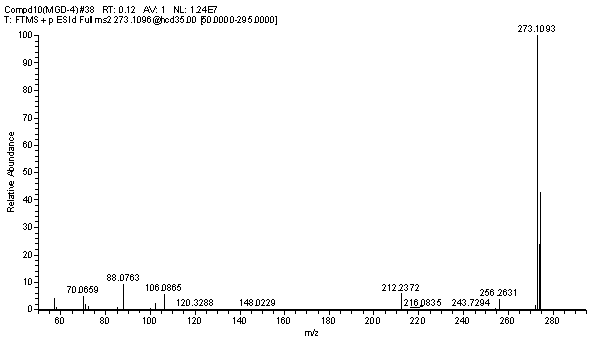
[0036] (1) The hapten was identified by nuclear magnetic resonance and liquid chromatography-mass spectrometry.
[0037] (2) The coupling result of the artificial antigen is identified by ultraviolet method, and the coupling ratio is calculated by using the concentration of small molecules and proteins in the conjugate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com