Method for preparing porous N-rich cobalt nitride with hard template method and application
A hard template method, cobalt nitride technology, applied in chemical instruments and methods, nitrogen compounds, catalyst activation/preparation, etc., can solve problems such as hindering the efficiency of oxygen evolution reaction, hindering the transmission of electrons from electrocatalyst to current collector, etc. Low cost, excellent catalytic activity, mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Step S1, preparing Co(OH) 2 NFWs precursor:
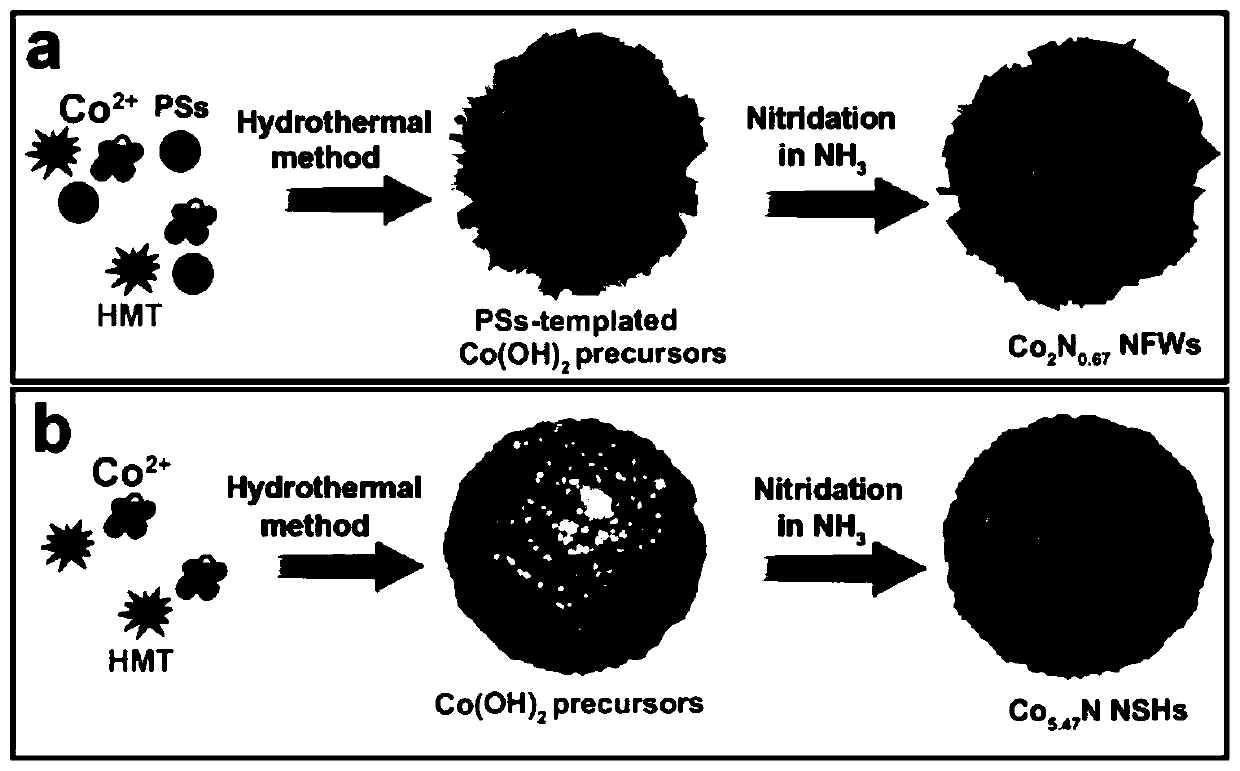
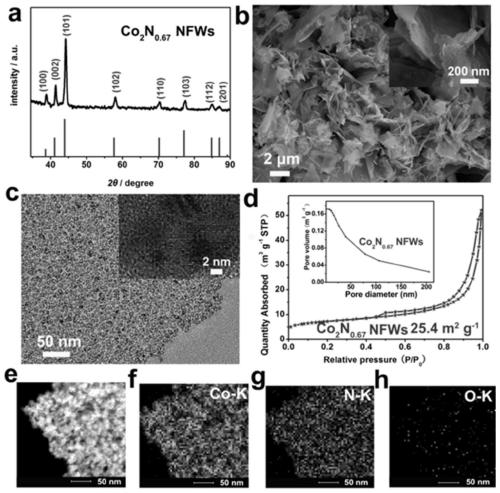
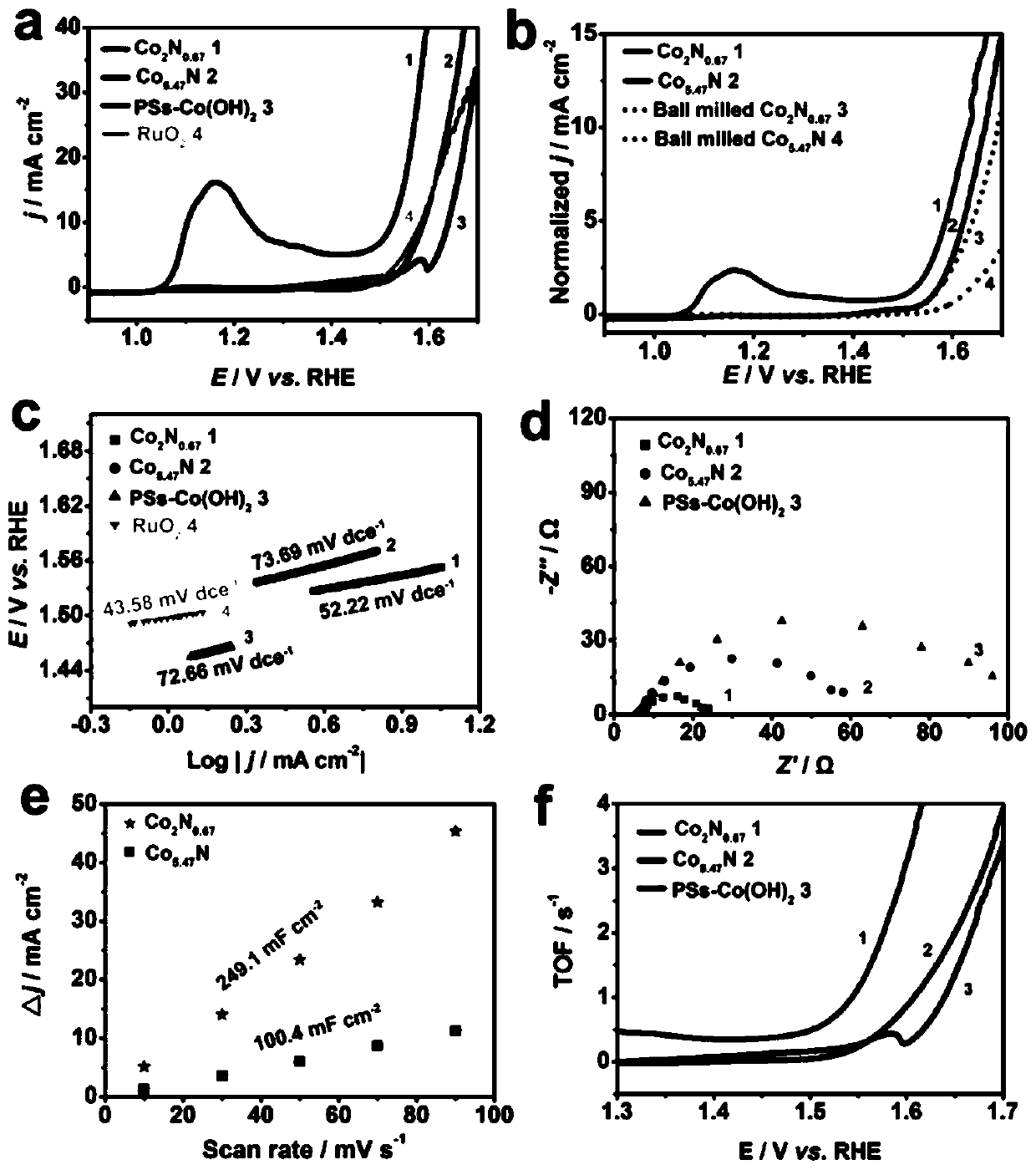
[0049] Co(OH) 2 The precursor of NFWs was synthesized by hydrothermal method without using sodium polystyrene sulfonate pellets (PSs) as a template, and 3.5 mmol Co(CH 3 COO) 2 4H 2 0. Add 7mmol of hexamethyltetramine (HMT) into 60mL of deionized water and stir for 2h, add the above mixed solution into 80mL of polytetrafluoroethylene-lined high-pressure axe, place in a high-temperature oven, and keep at 120°C for 12h. After the reaction is complete, after the high-pressure ax and the product are cooled to room temperature, the product is washed 4 times with deionized water and absolute ethanol, and dried overnight at 60°C to obtain Co(OH) 2 NFWs; while using the same method to prepare Co(OH) 2 NFWs precursor but using sodium polystyrenesulfonate pellets (PSs) as a template, 3.5mmol Co(CH 3 COO) 2 4H 2 O, 7mmol / L sodium polystyrene sulfonate pellets (PSs), 7mmol hexamethyltetramine (HMT) were added to 60mL deionized w...
Embodiment 2
[0055] Modify step S1 of Example 1 to prepare Co(OH) 2 NFWs precursor: Co(OH) 2 The precursor of NFWs was synthesized by hydrothermal method without using sodium polystyrene sulfonate pellets (PSs) as a template, and 2.5 mmol Co(CH 3 COO) 2 4H 2 0. 5 mmol of hexamethyltetramine (HMT) was added to 40 mL of deionized water and stirred for 1 h, the above mixed solution was added to a 60 mL polytetrafluoroethylene-lined high-pressure axe, placed in a high-temperature oven, and kept at 110 ° C for 11 h, After the reaction is complete, after the high-pressure ax and the product are cooled to room temperature, the product is washed three times with deionized water and absolute ethanol, and dried overnight at 40°C to obtain Co(OH) 2 NFWs; while using the same method to prepare Co(OH) 2 NFWs precursor but using sodium polystyrenesulfonate pellets (PSs) as a template, 2.5mmol Co(CH 3 COO) 2 4H 2 0, 5mmol / L sodium polystyrene sulfonate pellets (PSs), 5mmol hexamethyltetramine (HMT...
Embodiment 3
[0057] Modify step S1 of Example 1 to prepare Co(OH) 2 NFWs precursor: Co(OH) 2 The precursor of NFWs was synthesized by hydrothermal method without using sodium polystyrene sulfonate pellets (PSs) as a template, and 5 mmol Co(CH 3 COO) 2 4H 2 O. 10mmol of hexamethyltetramine (HMT) was added to 80mL of deionized water and stirred for 3h, the above mixed solution was added to a 100mL polytetrafluoroethylene-lined high-pressure axe, placed in a high-temperature oven, and kept at 130°C for 13h. After the reaction is complete, after the high-pressure ax and the product are cooled to room temperature, the product is washed 6 times with deionized water and absolute ethanol, and dried overnight at 80°C to obtain Co(OH) 2 NFWs; while using the same method to prepare Co(OH) 2 NFWs precursor but using sodium polystyrenesulfonate pellets (PSs) as a template, 5mmol Co(CH 3 COO) 2 4H 2 O, 10mmol / L sodium polystyrene sulfonate pellets (PSs), 10mmol hexamethyltetramine (HMT) were adde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com