Method for recycling byproduct p-toluene magnesium sulfonate to synthesize tenofovir
A technology of magnesium toluenesulfonate and tenofovir, applied in chemical instruments and methods, chemical/physical process, chemical/physical/physical chemical process, etc., can solve the problem of low content of sodium tosylate and sodium chloride, No effective treatment and comprehensive utilization, unfavorable large-scale production and environmental protection, etc., to achieve the effect of low energy consumption, recovery and recycling, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
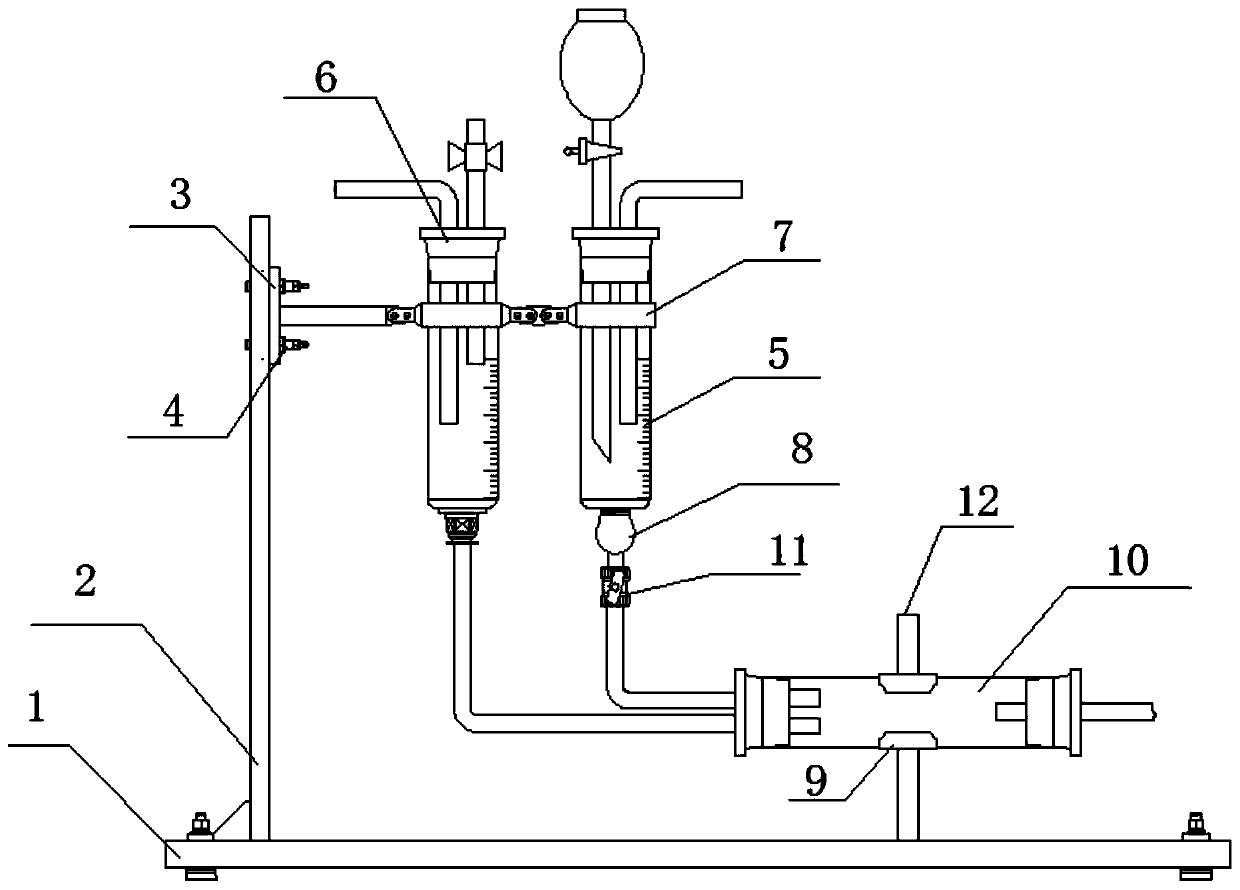
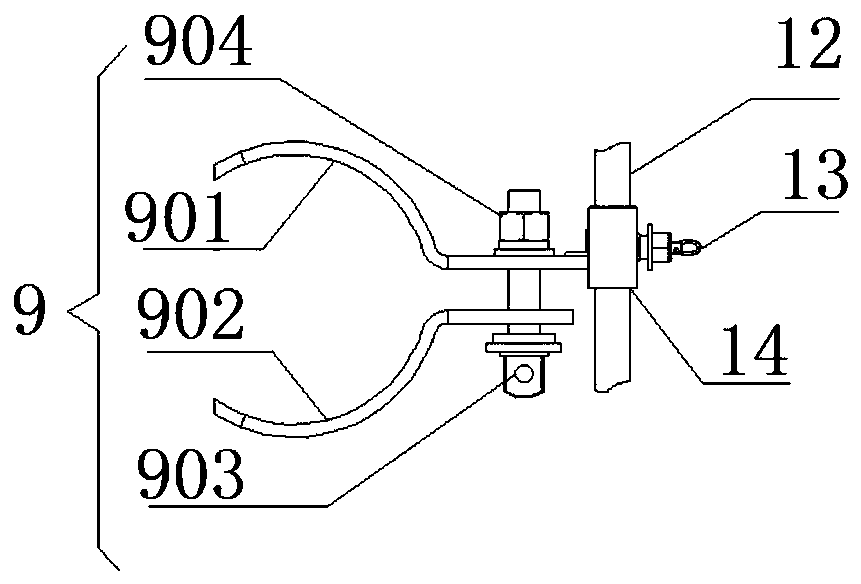
Image
Examples
Embodiment Construction
[0024] The following will clearly and completely describe the technical solutions in the embodiments of the present invention with reference to the accompanying drawings in the embodiments of the present invention. Obviously, the described embodiments are only some, not all, embodiments of the present invention.
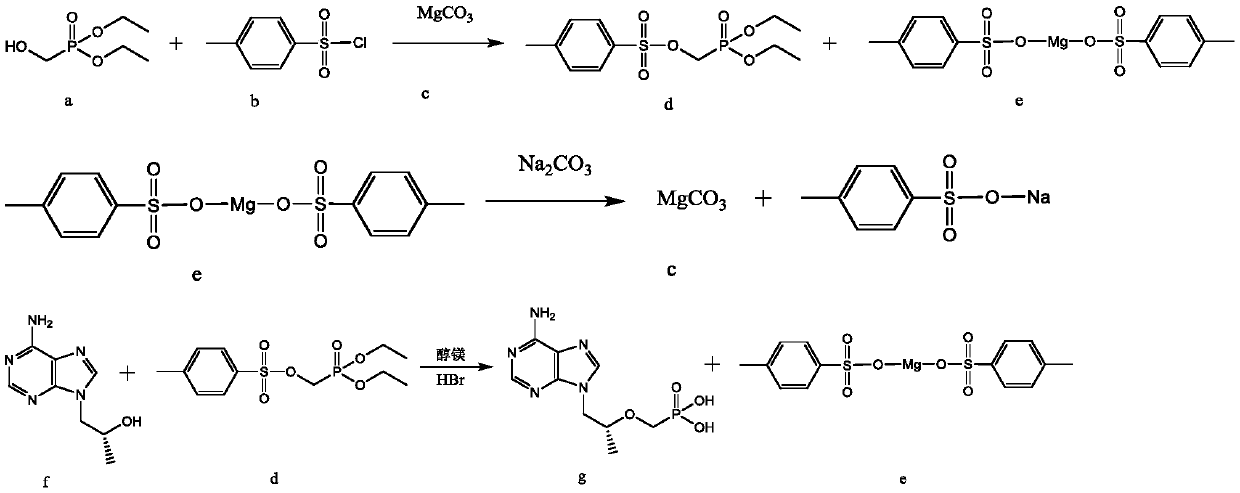
[0025] A method for synthesizing tenofovir by recycling by-product magnesium p-toluenesulfonate, the synthetic route of tenofovir is:
[0026]
[0027] Concrete synthetic steps are:
[0028] S1, compound a and compound b are purified under the action of C as an acid-binding agent, using water as a solvent to condense and react to obtain compound d, and at the same time produce by-product e. Compound a, compound b and compound c are tested in tenofovir Synthesized in a chamber preparation reaction device, and the equivalent ratio of compound a, compound b and compound c is 1:1.1-1.3:0.55-0.6; the reaction temperature is 55°C-70°C, and the reaction time is 20- 24 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com