Glutamate dehydrogenase mutants and application thereof
一种谷氨酸脱氢酶、突变体的技术,应用在催化2-羰基-4-丁酸制备L-草铵膦中的应用,谷氨酸脱氢酶突变体领域,能够解决2-羰基-4-(羟基甲基膦酰基)丁酸催化活力低等问题,达到良好催化效率、大工业应用前景、提高催化活力的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Pseudomonas putida-derived glutamate dehydrogenase (PpGluDH) directed evolution based on error-prone PCR
[0053] Step 1: Activation of PpGluDH recombinant bacteria and plasmid extraction
[0054] The Escherichia coli engineering bacteria carrying the pET-28a(+)-PpGluDH recombinant plasmid were activated and cultured in LB medium.
[0055] The specific formula of LB medium is: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, sterilized at 121°C for 20min, ready for use. The solid medium is LB medium with 2% agar added.
[0056] Streak the glycerol tube containing the PpGluDH recombinant bacteria onto a plate containing LB solid medium (50 μg / mL kanamycin), and culture it statically at 37° C. for 12 hours. Pick a single colony from the plate and insert it into 5 mL LB medium containing 50 μg / mL kanamycin, and culture at 37 °C and 200 rpm for 12 h. After obtaining the culture medium, extract the plasmid acco...
Embodiment 2
[0086] Example 2 Construction and Screening of Focused Saturation Mutation Library
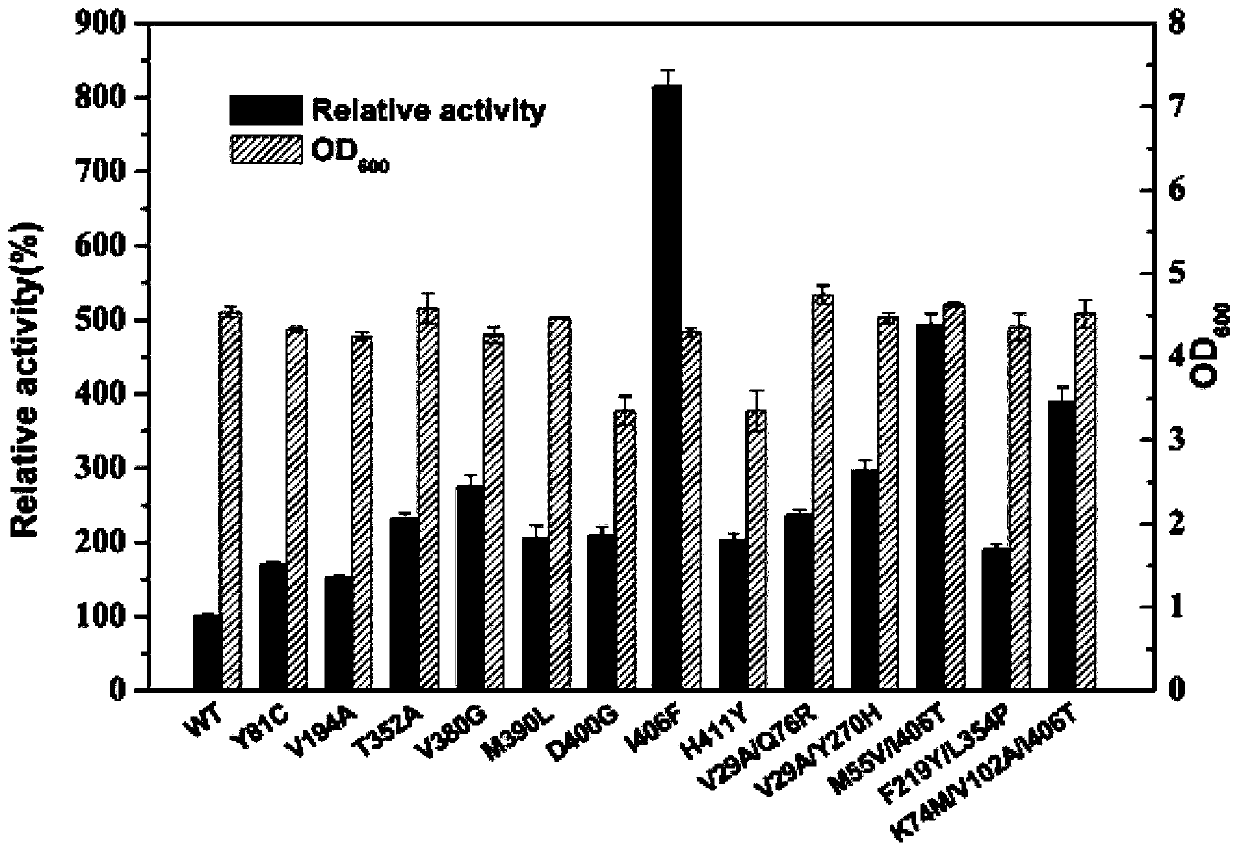
[0087] Analyzing the results of Example 1, saturation mutations were performed on amino acid residues 121, 123, 379, 383 and 402 of PpGluDH. Design primers (Table 6), use the pET-28a(+)-PpGluDH plasmid as a template, and use K402X-F, T121X / L123X-F or A379X / L383X-F and Aid-R as primer pairs for PCR to obtain linearized short fragments , and then use K402X-R, T121X / L123X-R or A379X / L383X-R and Aid-F as primer pairs to perform PCR to obtain linearized long fragments. Refer to the instruction manual of the recombination cloning kit (ClonExpress II One Step Cloning Kit) for subsequent DpnI digestion, gel recovery, recombination and transformation operations to construct a saturation mutation library at position 402 (K402X) and a combined mutation library at positions 121 and 123 (T121X / L123X), 379 and 383 combined mutation library (A379X / L383X) 3 mutation libraries.
[0088] Table 6 Primers used ...
Embodiment 3
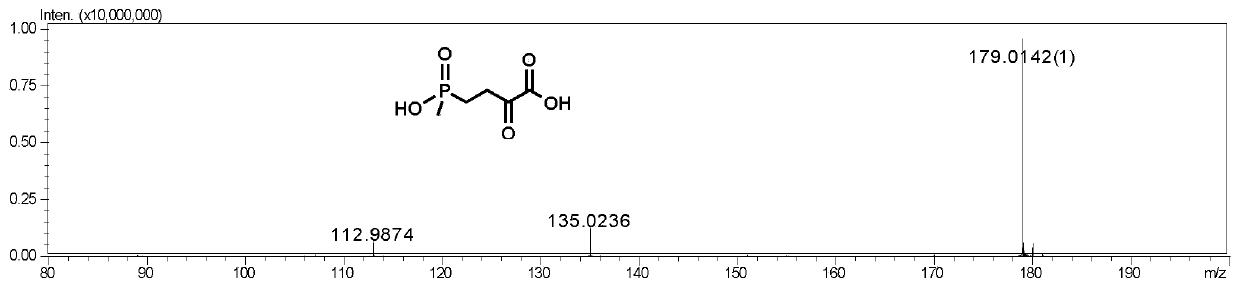
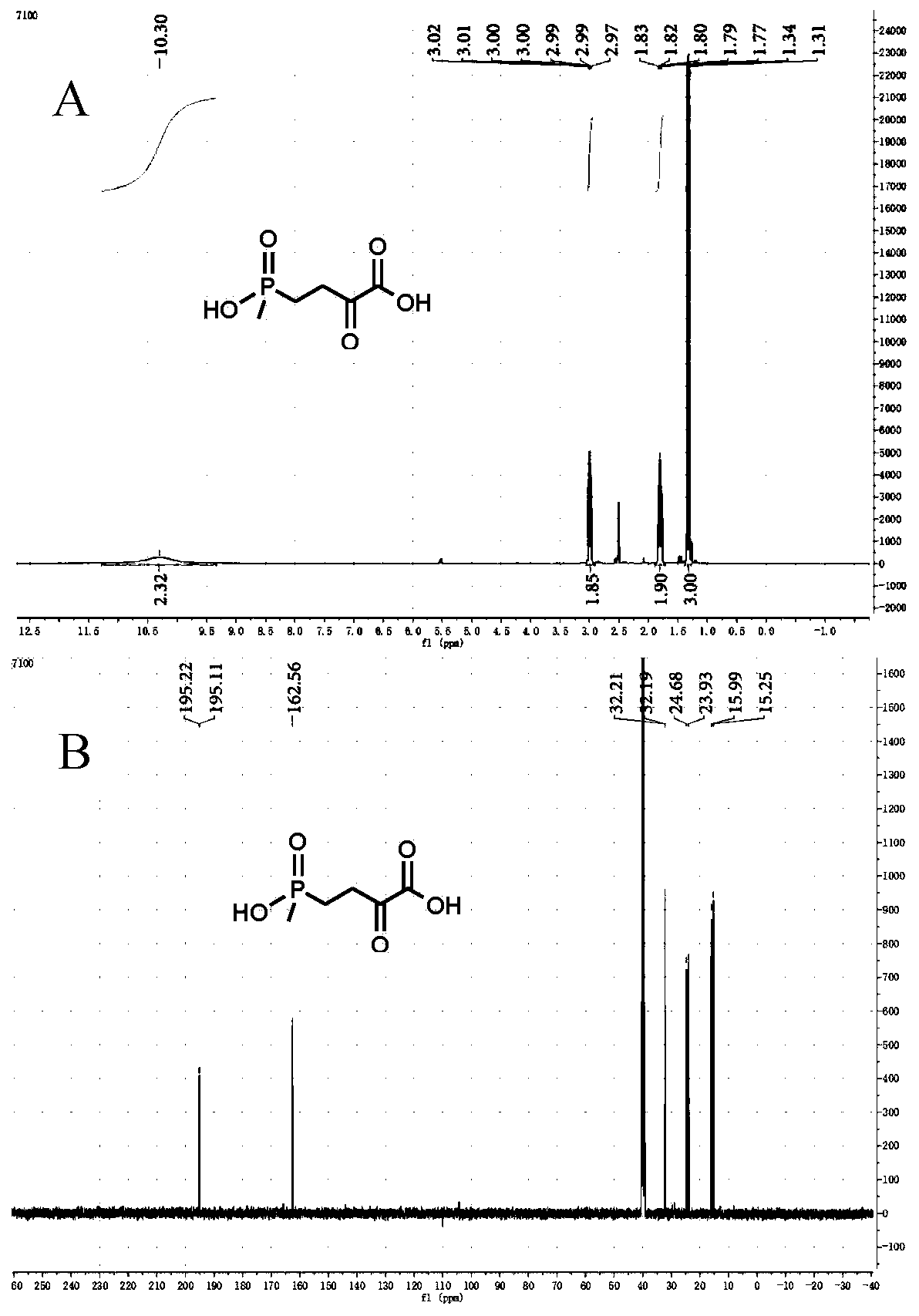
[0095] Example 3 Glutamate dehydrogenase wild-type (PpGluDH), glucose dehydrogenase dual enzyme coupling preparation of L-glufosinate-ammonium
[0096] Bacterial culture and crude enzyme solution preparation: After the glycerol tubes of engineered bacteria containing wild-type PpGluDH and glucose dehydrogenase (BsGDH-2M, SEQ ID NO.2) were activated by streaking on a plate, single colonies were inoculated into In 50 mL LB liquid medium containing 50 μg / mL kanamycin, shake culture at 37°C for 12 hours. Transfer to 1L fresh LB liquid medium also containing 50μg / ml Kan according to 2% inoculum size, and culture with shaking at 37°C until OD 600 When it reaches about 0.6, add IPTG to its final concentration of 0.5mM, and induce culture at 18°C for 16h. After the cultivation, the culture solution was centrifuged at 12000 g at 4° C. for 10 min, the bacteria were collected, and the cells were disrupted by ultrasonic to prepare the crude enzyme solution.
[0097] The reaction syste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com