NHPI-GPTMS-CoOX/SiO2 heterogeneous catalyst, applications and recovery method thereof
A heterogeneous catalyst and co-precipitation technology, applied in the field of heterogeneous catalysts, can solve problems such as poor stability and easy loss of NHPI, and achieve the effects of good stability, low equipment requirements, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
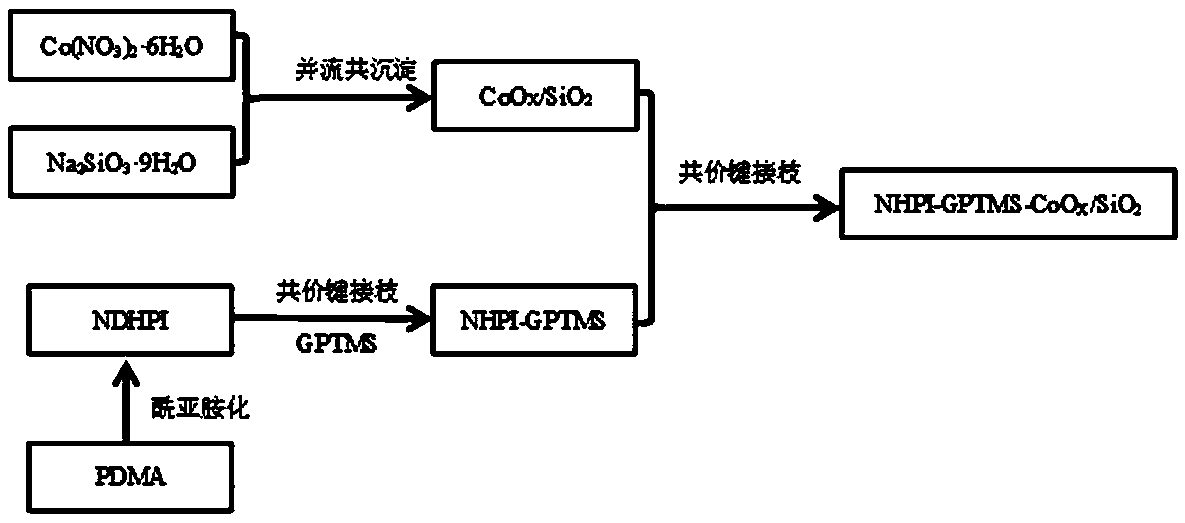
[0041] first press as figure 1 The procedure shown for the preparation of the heterogeneous catalyst NHPI-GPTMS-CoO X / SiO 2 , the preparation steps are:
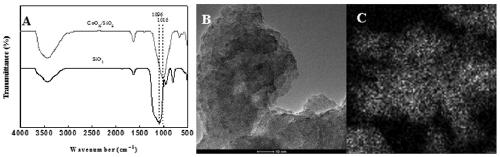
[0042] a) Weigh 2.91 g Co(NO 3 ) 2 ·6H 2 O and 2.84 g Na 2 SiO 3 9H 2 O was dissolved in 30 mL of deionized water, and added dropwise to 40 mL of deionized water, and stirred at 363 K for 0.5 h. Placed in a 363 K oven for 24 h. Filter and wash the filter cake repeatedly with deionized water; add 40 mL of n-butanol, mix well with the solid, and evaporate the n-butanol to dryness at 353 K; 3 h, CoO was obtained after grinding X / SiO 2 . like figure 2 CoO shown in A X / SiO 2 Infrared spectrum (A), high-resolution transmission electron microscopy (B) and cobalt element distribution map (C), CoO X / SiO 2 Infrared spectra of cobalt and silica supports form Si—O—Co bonds (1016 cm -1 ), figure 2 The high-resolution TEM image shown in B shows that CoO X / SiO 2 There are no obvious crystal morphology features ...
Embodiment 2
[0048] In this implementation, at first the solid-phase NHPI-GPTMS-CoO obtained after preparing benzaldehyde in Example 1 X / SiO 2 The recovered mixture of heterogeneous catalysts was separated, recovered and purified as follows: wash with a mixed solvent of diethyl ether and chloroform at a volume ratio of 1:1, and dry at 323 K for 24 h to obtain a recycled catalyst NHPI-GPTMS-CoO X / SiO 2 -R 1 .
[0049] The catalyst NHPI-GPTMS-CoO is recovered by this primary cycle X / SiO 2 -R 1 Prepare benzaldehyde by the method of embodiment 1, weigh 0.2 g NHPI-GPTMS-CoO X / SiO 2 -R 1 Place in a 50 mL polytetrafluoroethylene-lined autoclave, add 2 mmol of raw material toluene, and 40 mmol of solvent hexafluoroisopropanol, at a reaction temperature of 363 K, a magnetic stirring speed of 240 r / min and React for 5 h under the condition of oxygen pressure of 2.0 MPa. After the reaction, cool to room temperature and centrifuge to separate the solid and liquid phases. The liquid phase ...
Embodiment 3
[0051] In this example, the solid phase obtained after preparing benzaldehyde in Example 2 is NHPI-GPTMS-CoO X / SiO 2 -R 1 The recovery mixture of heterogeneous catalyst obtains the catalyst NHPI-GPTMS-CoO of secondary recovery by the separation recovery purification method of embodiment 2 X / SiO 2 -R 2 .
[0052] Then by the method of embodiment 2 with NHPI-GPTMS-CoO X / SiO 2 -R 2 To prepare benzaldehyde as a catalyst, the liquid phase reaction product was analyzed for benzaldehyde activity as shown in Table 1. The solid phase recovery mixture is used for the separation, recovery, purification and recycling of the next cycle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com