Continuous synthesis method of oseltamivir phosphate intermediate
A technology for oseltamivir phosphate and intermediates, which is applied in the field of continuous synthesis of oseltamivir phosphate intermediates, can solve problems such as safety accidents, equipment corrosion, strong corrosion, etc., to prevent safety accidents, avoid corrosion, The effect of ensuring production safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
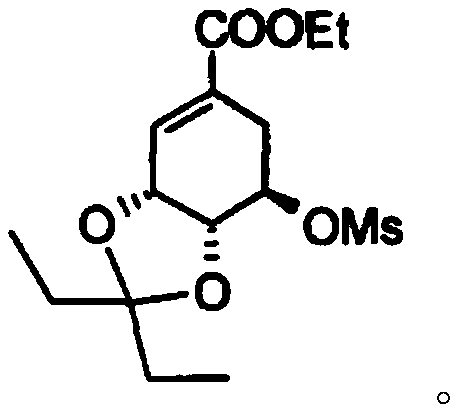
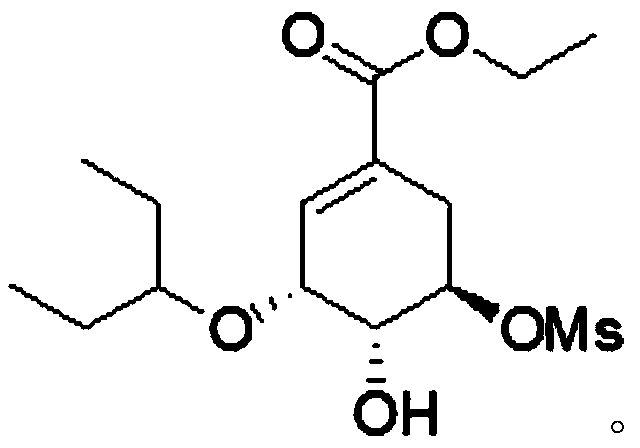
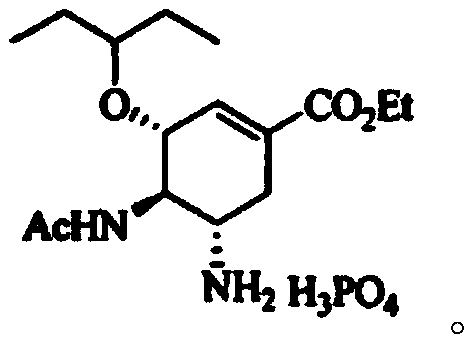
[0031] Add mesylate (compound II, 5.5g, 15.8mmol) and triethylsilane (2.38g, 20.5mmol) into dichloromethane (32g) and stir to dissolve to obtain solution 1. Titanium tetrachloride (3.3 g, 17.3mmol) was added into dichloromethane (20g) to obtain solution 2, solution 1 was pumped into the microchannel reaction with pump A at a speed of 1g / min and solution 2 was simultaneously pumped into the microchannel reaction with pump B at a speed of 0.58g / min The reaction is carried out in the microchannel of the device (coiled tube, length 26m, equivalent diameter 4mm), the temperature is set to -15°C, the retention time of the reaction solution is 20min, and the continuous reaction solution is obtained. After the reaction is completed by HPLC detection, the continuous reaction solution The solution was poured into ice water to extract the liquid separation, and then saturated NaHCO 3 Washing, separating, collecting the organic phase, drying and concentrating to obtain 5.5 g o...
Embodiment 2
[0034]
[0035] Add mesylate (compound II, 5.5g, 15.8mmol) and triethylsilane (2.75g, 23.7mmol) into dichloromethane (35.8g) and stir to dissolve to obtain solution 1. Titanium tetrachloride ( 3.3g, 17.3mmol) was added in dichloromethane (18g) to obtain solution 2, solution 1 was pumped into the microchannel reaction with pump A at a speed of 2g / min and solution 2 was simultaneously pumped into the microchannel reaction with pump B at a speed of 1g / min React in the microchannel (coil pipe, length 10m, equivalent diameter 5mm), temperature is set to -20 ℃, and the retention time of reaction liquid is 30min, obtains continuous reaction liquid, HPLC detects that reaction is finished, and this continuous reaction The solution was poured into ice water to extract the liquid separation, and then saturated NaHCO 3 Washing, separating, collecting the organic phase, drying, and concentrating to obtain 5.2 g of compound I.
[0036] After calculation, the yield of compound I in Examp...
Embodiment 3
[0038]
[0039] Add mesylate (compound II, 5.5g, 15.8mmol) and triethylsilane (1.83g, 15.8mmol) into dichloromethane (16.8g) and stir to dissolve to obtain solution 1. Titanium tetrachloride ( 3.3g, 17.3mmol) was added to dichloromethane (16.8g) to obtain solution 2, and solution 1 was pumped simultaneously with pump A at a rate of 0.5g / min and solution 2 was simultaneously pumped with pump B at a rate of 0.5g / min Enter in the microchannel of microchannel reactor (coil pipe, long 50m, equivalent diameter 1mm) to react, temperature is set to-20 ℃, and the retention time of reaction solution is 10min, obtains continuous reaction solution, HPLC detects that reaction is finished, and this The continuous reaction solution was poured into ice water for extraction and separation, and then saturated NaHCO 3 Wash, separate, collect the organic phase, dry and concentrate to obtain 5.0 g of compound I.
[0040] After calculation, the yield of compound I in Example 3 was 90.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com