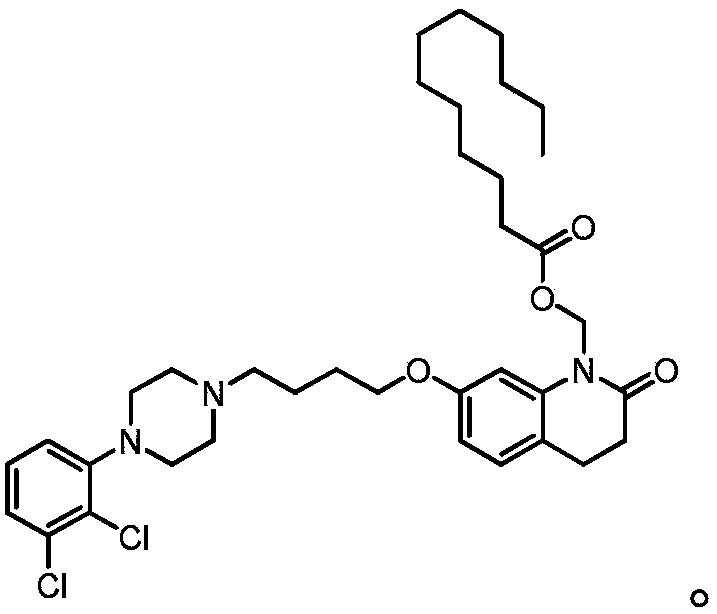
Preparation method of aripiprazole lauroxil
A technology of lauroyl aripiprazole and aripiprazole, which is applied in the field of drug synthesis, can solve the problems of inconvenient industrial production, difficulty in complete desolventization, and high risk, and achieve the goal of shortening drying time and reducing organic solvents and water Residue, Residue reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Intermediate 7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-1-(hydroxymethyl)-3,4-dihydroquinoline-2 Preparation of (1H)-ketone (hydroxymethyl aripiprazole):
[0057] Aripiprazole (45g, 100.63mmol), aqueous formaldehyde (37% mass concentration, 120mL), triethylamine (7mL, 50.32mmol, 0.50eq molar equivalent to aripiprazole) and N,N -The mixture of dimethylformamide (135mL) was heated to 80°C, reacted for 2.5h, cooled to room temperature, and suction filtered; the filter cake was washed twice with acetonitrile, each time 30mL, and air-dried to obtain 53.6g of product, the yield It is 96% and the purity is 90.12%.
[0058] 1 H NMR (CDCl 3 ,400MHZ) δ1.62~1.78(m, 2H), 1.77~1.91(m, 2H), 2.43~2.77(m, 8H), 2.82(t, 2H), 3.11(s, 4H), 3.71(s, 1H), 4.01(t, 2H), 5.33(s, 2H), 6.57(dd, 1H), 6.86(d, 1H), 6.96(m, 1H), 7.04(d, 1H), 7.10~7.20(m , 2H); m / z (M+H)=478.
Embodiment 2
[0060] Intermediate 7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-1-(hydroxymethyl)-3,4-dihydroquinoline-2 Preparation of (1H)-ketone (hydroxymethyl aripiprazole):
[0061] Aripiprazole (26g, 58.15mmol), aqueous formaldehyde (37% mass concentration, 90mL), triethylamine (4mL, 29.07mmol, 0.50eq molar equivalent to aripiprazole) and N,N -The mixture of dimethylformamide (78mL) was heated to 80°C, reacted for 2.5h, cooled to room temperature, filtered with suction, the filter cake was washed twice with acetone, 26mL each time, and air-dried to obtain 26.1g product, the yield It is 94%, and the purity is 91.34%.
[0062] The product obtained is substantially the same as the hydrogen spectrum information in Example 1.
Embodiment 3
[0064] Intermediate 7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-1-(hydroxymethyl)-3,4-dihydroquinoline-2 Preparation of (1H)-ketone (hydroxymethyl aripiprazole):
[0065] Aripiprazole (26g, 58.15mmol), aqueous formaldehyde (37% mass concentration, 90mL), triethylamine (4mL, 29.07mmol, 0.50eq molar equivalent to aripiprazole) and N,N -The mixture of dimethylformamide (78mL) was heated to 80°C, reacted for 2.5h, cooled to room temperature, filtered with suction, the filter cake was washed twice with ethanol, 26mL each time, and air-dried to obtain 24.2g product, the yield It is 87.2%, and the purity is 91.05%.
[0066] The product obtained is substantially the same as the hydrogen spectrum information in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com