Charged magnetic hydrophobic material and preparation method and application thereof in separation of micro-fine-particle minerals
A hydrophobic material and electromagnetic technology, applied in the direction of solid separation, flotation, etc., can solve the problems of low ore concentrate grade, severe, and ineffective recovery of useful minerals, and achieve simple preparation methods, high recovery rates, and high-efficiency separation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
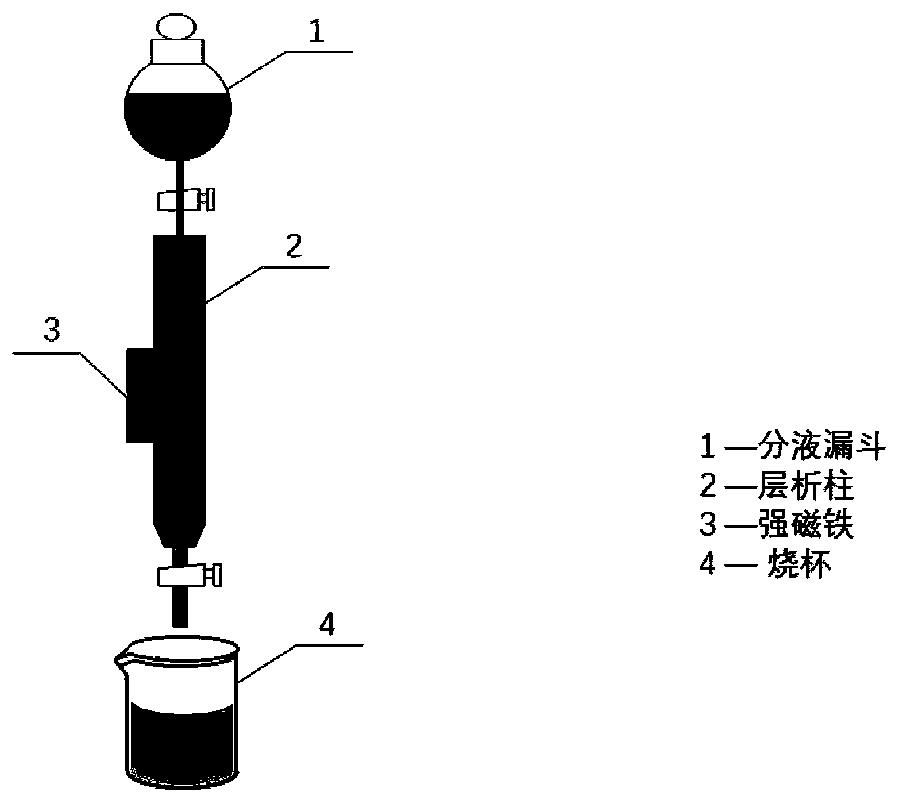
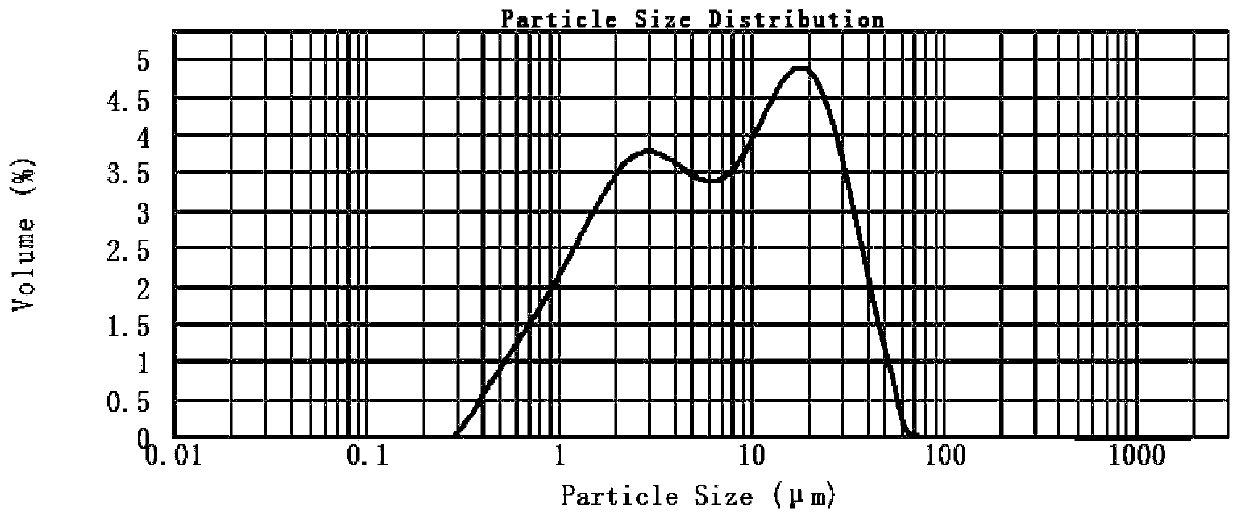
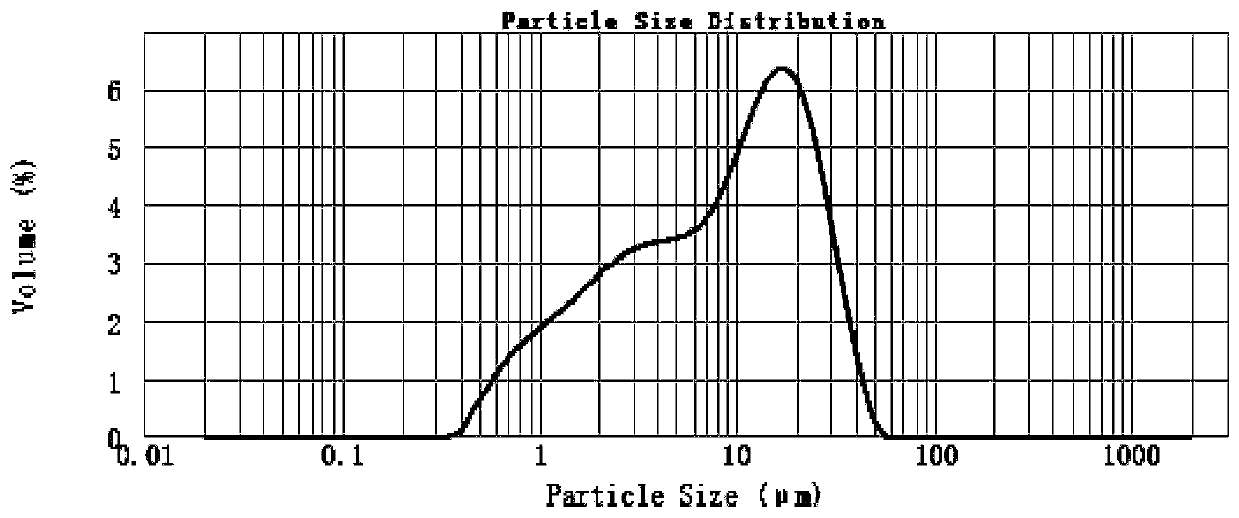
Image
Examples
Embodiment 1
[0045] Preparation of the modified magnetic carrier: prepare a solution of benzyl hydroxamic acid with a concentration of 0.01mol / L and a solution of lead nitrate with a concentration of 0.02mol / L. Take 20 mL each and mix in a beaker (adjust the pH of the solution to 9.5), add 5 g of magnetite with a particle size ranging from 400 mesh to 600 mesh, and stir for 30 minutes at a speed of 200 r / min by a mechanical stirrer to make the magnetite The ore is in full contact with the lead metal complex of benzohydroxamic acid. The modified magnetite is filtered and dried in a vacuum oven to obtain the modified magnetic carrier.
[0046] Configuration of sodium oleate solution: Weigh 0.2436g sodium oleate in a beaker, add a little deionized water, heat to completely dissolve sodium oleate, transfer the solution to a 100mL volumetric flask, cool to room temperature, and then settle to volume to obtain a concentration of 8×10 -3 mol / L sodium oleate solution for use.
[0047] The schee...
Embodiment 2
[0051] Preparation of the modified magnetic carrier: prepare a solution of benzyl hydroxamic acid with a concentration of 0.01mol / L and a solution of lead nitrate with a concentration of 0.02mol / L. Take 20mL each and mix in a beaker (adjust the solution pH=10), add 5g of magnetite with a particle size ranging from 400 mesh to 600 mesh, and stir for 10 minutes at a speed of 200r / min by a mechanical stirrer to make the magnetite The ore is in full contact with the lead metal complex of benzohydroxamic acid. The modified magnetite is filtered and dried in a vacuum oven to obtain the modified magnetic carrier.
[0052] Configuration of sodium oleate solution: Weigh 0.2436g sodium oleate in a beaker, add a little deionized water, heat to completely dissolve sodium oleate, transfer the solution to a 100mL volumetric flask, cool to room temperature, and then settle to volume to obtain a concentration of 8×10 -3 mol / L sodium oleate solution for use.
Embodiment 3
[0057] Preparation of the modified magnetic carrier: prepare a solution of benzyl hydroxamic acid with a concentration of 0.01 mol / L and a solution of calcium chloride with a concentration of 0.02 mol / L. Take 20 mL each and mix in a beaker (adjust the pH of the solution to 9.5), add 5 g of magnetite with a particle size ranging from 400 mesh to 600 mesh, and stir for 30 minutes at a speed of 200 r / min by a mechanical stirrer to make the magnetite The ore and the calcium metal complex of benzohydroxamic acid fully contact and react. The modified magnetite is filtered and dried in a vacuum oven to obtain the modified magnetic carrier.
[0058] Configuration of sodium oleate solution: Weigh 0.2436g sodium oleate in a beaker, add a little deionized water, heat to completely dissolve sodium oleate, transfer the solution to a 100mL volumetric flask, cool to room temperature, and then settle to volume to obtain a concentration of 8×10 -3 mol / L sodium oleate solution for use.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com