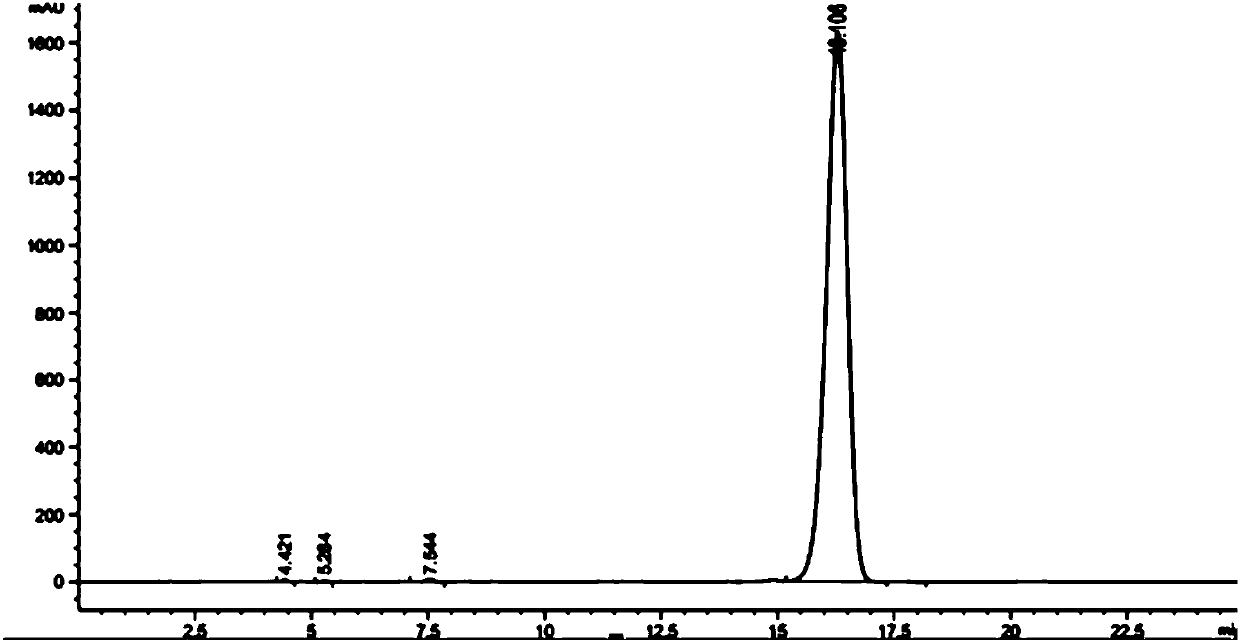
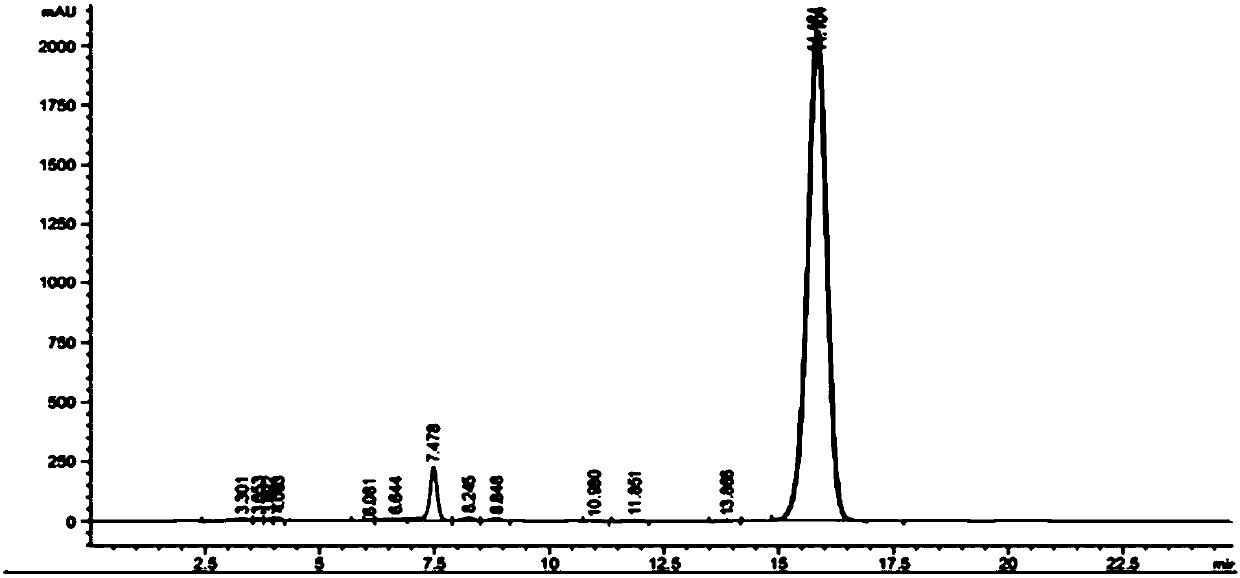
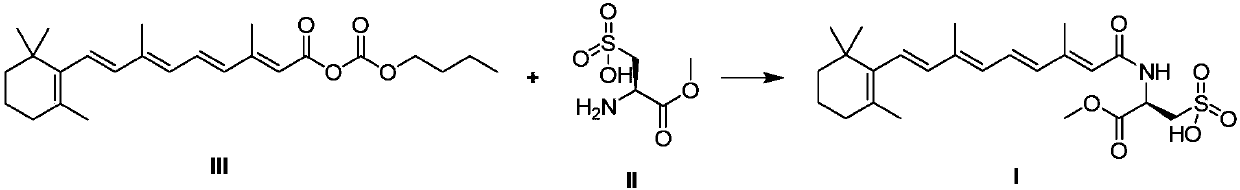
Preparation method of sodium salt of N-(all trans-retinol)-L-cystathionine methyl ester
A technology of methyl cystylate and all-trans, which is applied in the direction of organic chemistry, can solve the problems of low product yield, long reaction time, and low product purity, and achieve high product yield, improved product purity, and high product purity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of all-trans retinoic acid butyric anhydride reaction solution: In a 100mL four-necked flask, add all-trans retinoic acid (2.7g, 9mmol), 18mL tetrahydrofuran, 36mL acetonitrile, and 1.5mL triethylamine in sequence at room temperature , placed in a low-temperature cooling tank, mechanically stirred, and when the reaction system was cooled to -20°C, slowly added butyl chloroformate (1.26mL, 9.9mmol), after the addition was complete, the reaction was continued for 30min to obtain all-trans retinoic acid butyric anhydride The reaction solution.
[0048] Preparation of the sodium salt of N-(all-trans-retinoyl)-L-cysteinesulfonate methyl ester: in a 250mL four-neck flask, add L-cysteinesulfonate methyl ester (2.97g , 13.5mmol), 60mL N,N-dimethylformamide, 2.4mL triethylamine, mechanical stirring, room temperature, after stirring for 30min, began to slowly add the all-trans retinoic acid butyric anhydride reaction solution obtained above. After the dropwise additi...
Embodiment 2
[0054] Preparation of all-trans retinoic acid butyric anhydride reaction solution: In a 100mL four-necked flask, add all-trans retinoic acid (2.7g, 9mmol), 18mL tetrahydrofuran, 36mL acetonitrile, and 1.5mL triethylamine in sequence at room temperature , placed in a low-temperature cooling tank, mechanically stirred, and when the reaction system was cooled to -20°C, butyl chloroformate (1.26mL, 9.9mmol) was slowly added. After the addition was complete, the reaction was continued for 30min. Obtain all-trans retinoic acid butyric anhydride reaction liquid.
[0055] Preparation of the sodium salt of N-(all-trans-retinoyl)-L-cysteinesulfonate methyl ester: in a 250mL four-neck flask, add L-cysteinesulfonate methyl ester (2.97g , 13.5mmol), 90mL N,N-dimethylformamide, 2.4mL triethylamine, mechanical stirring, room temperature, after stirring for 30min, began to slowly add the all-trans retinoic acid butyric anhydride reaction solution obtained above. After the dropwise addition, ...
Embodiment 3
[0057] Preparation of all-trans retinoic acid butyric anhydride reaction solution: In a 100mL four-necked flask, add all-trans retinoic acid (2.7g, 9mmol), 18mL tetrahydrofuran, 36mL acetonitrile, and 1.5mL triethylamine in sequence at room temperature , placed in a low-temperature cooling tank, mechanically stirred, and when the reaction system was cooled to -20°C, butyl chloroformate (1.26mL, 9.9mmol) was slowly added. After the addition was complete, the reaction was continued for 30min. Obtain all-trans retinoic acid butyric anhydride reaction liquid.
[0058] Preparation of the sodium salt of N-(all-trans-retinoyl)-L-cysteinesulfonate methyl ester: in a 250mL four-neck flask, add L-cysteinesulfonate methyl ester (2.97g , 13.5mmol), 60mL N,N-dimethylformamide, 3mL diisopropylethylamine, mechanical stirring, room temperature, after stirring for 30min, began to slowly add the all-trans retinoic acid butyric anhydride reaction solution obtained above. After the dropwise addi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com