Cloning expression and application of fusion bursin
A technology of tripeptide bursin and fusion peptide, which is applied in the field of molecular biology, can solve the problems of finding ALV strains and the loss of breeding industry, etc., and achieve good market application prospects, strong inhibition of retroviruses, and good immune enhancement The effect of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
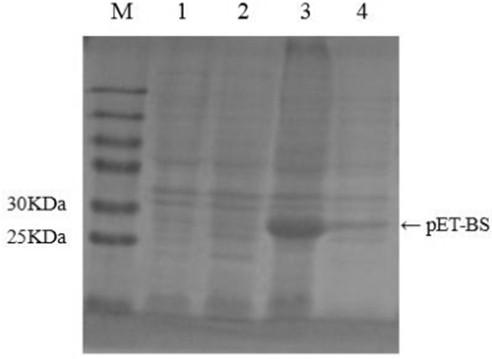
[0024] Example 1 Cloning expression of fusion tripeptide bursin
[0025] 1. Involvement and synthesis of primers
[0026] According to the specificity of foreign protein encoded by Escherichia coli, a tandem tetrapeptide BS (8) was designed and synthesized, and a fusion peptide sequence was added at the end. The amino acid sequence of the fusion BS was GSPRM KNPY KNPY KNPY KNPY KNPY KNPY KNPY KNPYHMGGGG SKLQRLMEDICLPRWGCLWEDDF, the core of which was The nucleotide sequence is: ATGAA AAACC CGTAT AAAAA CCCGTATAAA AACCC GTATA AAAAC CCGTA TAAAA ACCCG TATAA AAACC CGTAT AAAAA CCCGT ATAAAAACCC GTAT C ATAT G GGCGG CGGCG GCAGC AAGCT T CAGC GCCTG ATGGA AGATA TTTGC CTGCCGCGTT GGGGC TGCCT GTGGG AAGAT GATTT TTAAT AA was sent to Shanghai Sangon Bioengineering Co., Ltd. for synthesis and cloned into the pET-32a plasmid to construct the recombinant plasmid pET-BS. In addition, according to the published sequence of pET-32a and the designed and synthesized recombinant pET-BS, specific pri...
Embodiment 2
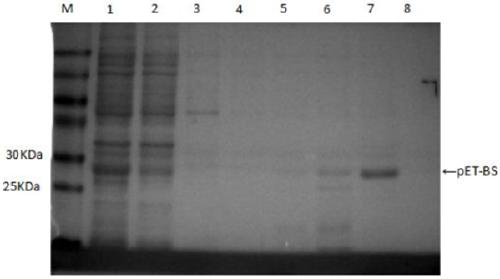
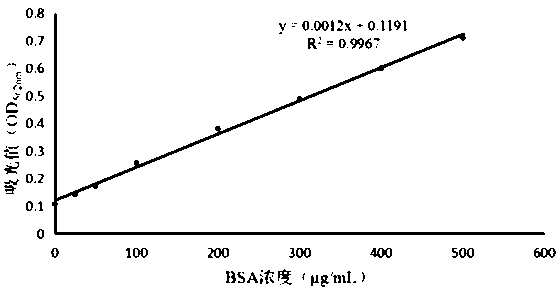
[0039] Example 2 Purification of Soluble Protein and BCA Quantification
[0040] According to the purification kit ( Protein Iso TM Ni-NTA Resin) instructions. The specific purification steps are as follows:
[0041] (1) Cleaning of the chromatographic column: first use ultrapure water to rinse the chromatographic column so that the negative of the chromatographic column is close to the bottom of the column.
[0042] (2) Column packing: Resuspend the medium, add an appropriate amount of medium to the chromatography column according to the amount of BS protein to be purified, let it stand, and discard the effluent.
[0043] (3) Equilibration: Equilibrate the column with 8 times the volume of the column packing medium in soluble equilibration buffer (containing 10 mM imidazole), and discard the effluent.
[0044] (4) Sample loading: Slowly add the BS soluble protein after high-speed centrifugation into the chromatography column, and collect the effluent.
[0045] (5) Washin...
Embodiment 3
[0050] Example 3 Establishment of an animal model of ALV congenital infection
[0051] (1) Culture of ALV and titration of virus infectivity (TCID50)
[0052] The ALV-FJ15HT0 strain was inoculated on a monolayer of DF-1 cells, placed in an incubator containing 5% CO2 at 37 °C, and collected after one week. First, it was frozen and thawed three times in a row, then filtered with a 0.22 μm filter, and finally frozen. Store in a -80°C freezer. The determination of ALV TCID50 selects the ALV p27 antigen detection kit produced by IDEXX Company. The assay was performed according to the kit instructions.
[0053] (2) Incubation of chicken embryos
[0054] The purchased SFP chicken embryos were placed in a fully automatic incubator. The specific incubation temperature and humidity for different embryo ages are shown in Table 3:
[0055] Table 3 Conditions for incubation of chicken embryos
[0056]
[0057] (3) SPF chicken embryo yolk sac inoculation
[0058] At the 7th embryo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com