Catalyst for synthesizing dimethyl carbonate and preparation method thereof
A technology of dimethyl carbonate and catalyst, applied in the field of catalyst for synthesizing dimethyl carbonate and its preparation field, can solve problems such as insufficient catalytic performance, achieve the effects of good activity and selectivity, simple operation of recovery process, and reduced use cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
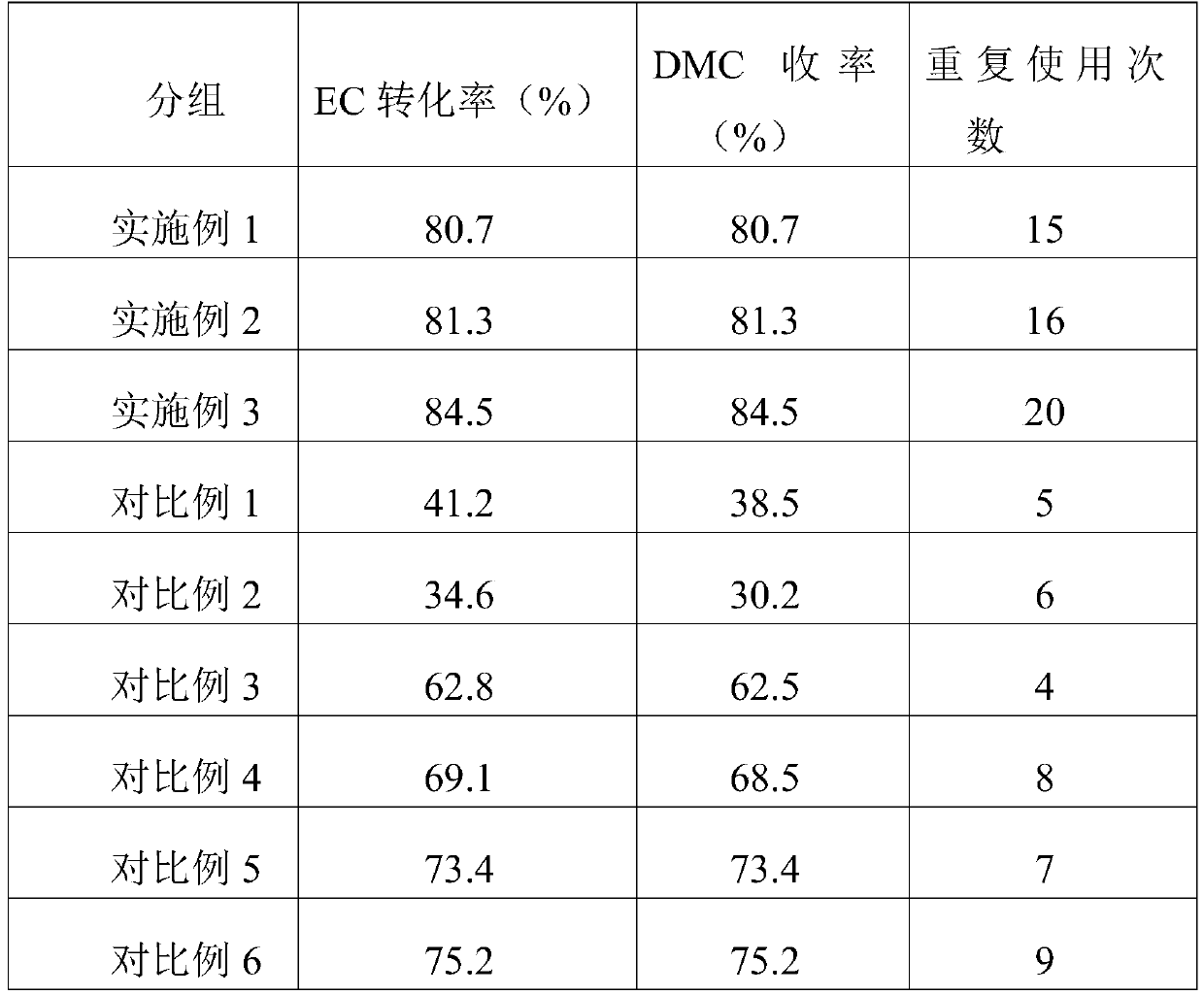
Examples
Embodiment 1
[0062]Prepare 100 mL of mixed solution A of zinc nitrate and ferric nitrate with deionized water, wherein the molar ratio of zinc ions to aluminum ions is 2. Then prepare 100mL of sodium carbonate solution B. Under continuous stirring, add solutions A and B dropwise into the reactor; then add an appropriate amount of sodium hydroxide solution with a concentration of 2mol / L to keep the pH value during the precipitation process at 9.5; The product was aged overnight at 55° C. in the mother liquor; the precipitate was separated by filtration, washed several times with water, and dried at 100° C. for 12 hours to obtain the hydrotalcite-like precursor.
[0063] Account for 6.5wt% of catalyst weight by the active component in the final catalyst, Bi 2 o 3 : KOH (weight ratio) = 1:2 to prepare the impregnation solution of bismuth nitrate and potassium hydroxide, wherein the concentration of bismuth ions is 0.2mol / L, and the concentration of KOH is 0.5mol / L. The zinc aluminum hydrot...
Embodiment 2
[0065] Prepare 100 mL of mixed solution A of zinc nitrate and ferric nitrate with deionized water, wherein the molar ratio of zinc ions to aluminum ions is 4. Then prepare 100mL of sodium carbonate solution B. Under continuous stirring, add solutions A and B dropwise into the reactor; then add an appropriate amount of sodium hydroxide solution with a concentration of 2mol / L to keep the pH value during the precipitation process at 10.5; after the precipitation is complete, the precipitation The product was aged overnight at 65° C. in the mother liquor; the precipitate was separated by filtration, washed several times with water, and dried at 100° C. for 12 hours to obtain the hydrotalcite-like precursor.
[0066] Account for 9.5wt% of catalyst weight by active component in the final catalyst, Bi 2 o 3 : KOH (weight ratio) = 1:4 to prepare the impregnation solution of bismuth nitrate and potassium hydroxide, wherein the concentration of bismuth ions is 0.8mol / L, and the concen...
Embodiment 3
[0068] Prepare 100 mL of mixed solution A of zinc nitrate and ferric nitrate with deionized water, wherein the molar ratio of zinc ions to aluminum ions is 3. Then prepare 100mL of sodium carbonate solution B. Under continuous stirring, add solutions A and B dropwise into the reactor; then add an appropriate amount of sodium hydroxide solution with a concentration of 2mol / L to keep the pH value during the precipitation process at 10; The product was aged overnight at 60° C. in the mother liquor; the precipitate was separated by filtration, washed several times with water, and dried at 100° C. for 12 hours to obtain the hydrotalcite-like precursor.
[0069] Account for 7wt% of catalyst weight by the active component in the final catalyst, Bi 2 o 3 : KOH (weight ratio) = 1:3 to prepare the impregnation solution of bismuth nitrate and potassium hydroxide, wherein the concentration of bismuth ions is 0.5mol / L, and the concentration of KOH is 1.2mol / L. The zinc aluminum hydrotal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com