Rb ion substituted OMS-2 catalyst as well as preparation method and application thereof
An OMS-2, ion substitution technology, applied in separation methods, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of low low-temperature catalytic activity and limited improvement range, and achieve improved activity, reduced production costs, Easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) The potassium permanganate that takes 0.02mmol is dissolved in the distilled water of 100mL, dissolves under magnetic stirrer;
[0029] (2) 0.001mmol of rubidium nitrate (the Rb salt can be one or more of rubidium nitrate, rubidium chloride, rubidium sulfate, rubidium acetate, rubidium fluoride, rubidium carbonate, rubidium iodide) and 0.5 The sulfuric acid of mL (this acid can be the mixing of one or more in nitric acid, hydrochloric acid, sulfuric acid, phosphoric acid, hypochlorous acid, citric acid, acetic acid, hydrofluoric acid, oxalic acid) joins step (1) middle solution successively, mix;
[0030] (3) Add 0.01 mmol of manganese nitrate to the solution of step (2), mix well, then seal the mouth of the beaker with plastic wrap, and react at 70°C for 24 hours.
[0031] (4) After the reaction is completed, the substrate obtained by the above reaction is washed with distilled water until the conductivity is 100 μs / cm; the washed sample is dried at 50°C to obtain...
Embodiment 2
[0037] (1) The potassium permanganate that takes 0.02mmol is dissolved in the distilled water of 100mL, dissolves under magnetic stirrer;
[0038] (2) The sulfuric acid of the rubidium nitrate of 0.003mmol and the sulfuric acid of 0.5mL successively joins the solution in step (1), mixes;
[0039] (3) Add 0.01 mmol of manganese nitrate to the solution of step (2), mix well, then seal the mouth of the beaker with plastic wrap, and react at 70°C for 24 hours.
[0040] (4) After the reaction is finished, the substrate obtained by the above reaction is washed with distilled water until the conductivity is 100 μs / cm; the washed sample is dried at 50°C to obtain the 0.1Rb-OMS-2 catalyst.
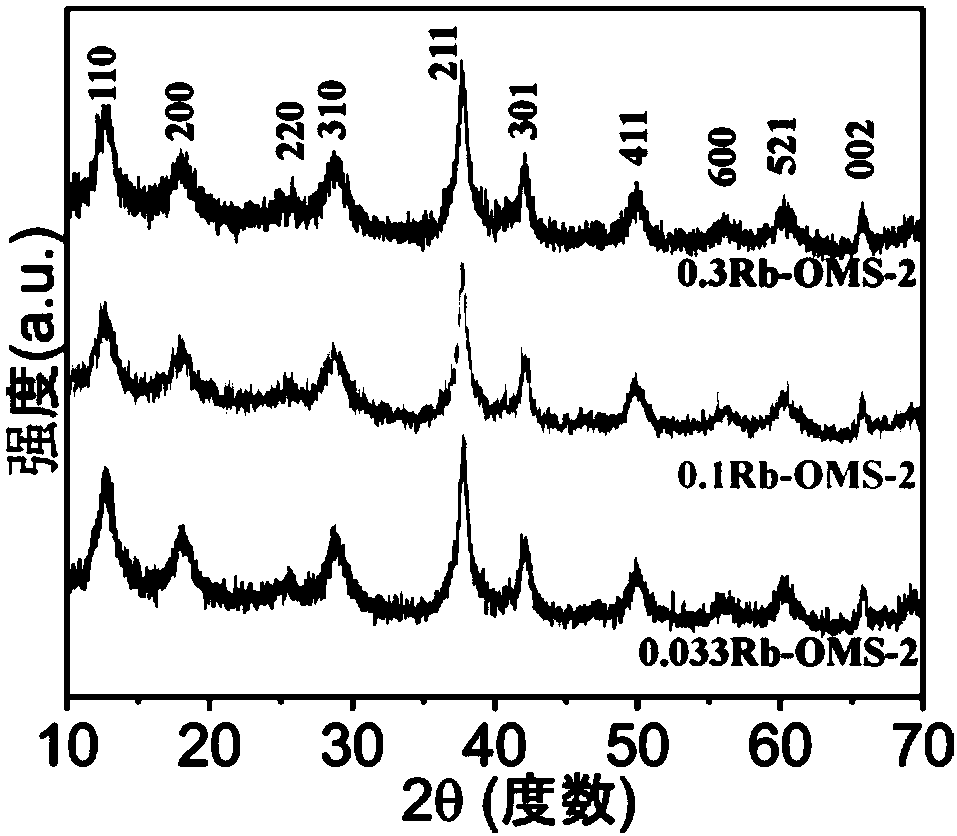
[0041] Catalyst phase identification: the XRD collection of illustrative plates of the catalyst that embodiment 2 obtains is as figure 1 As shown, it is a cryptopotassium manganese ore structure.
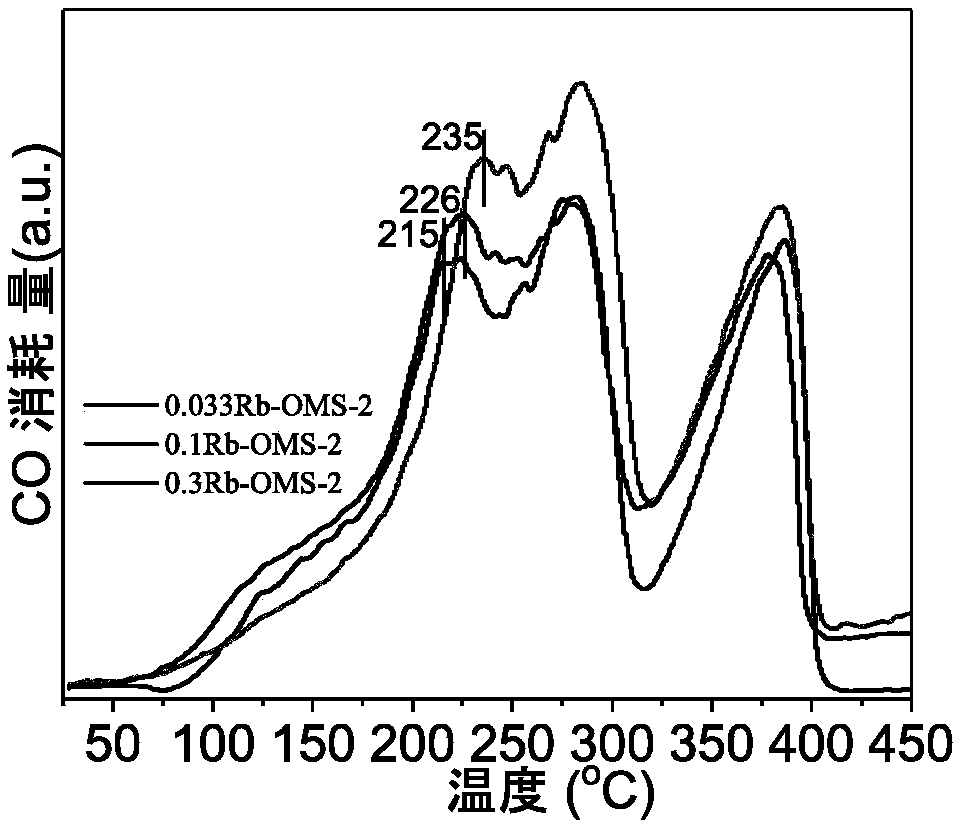
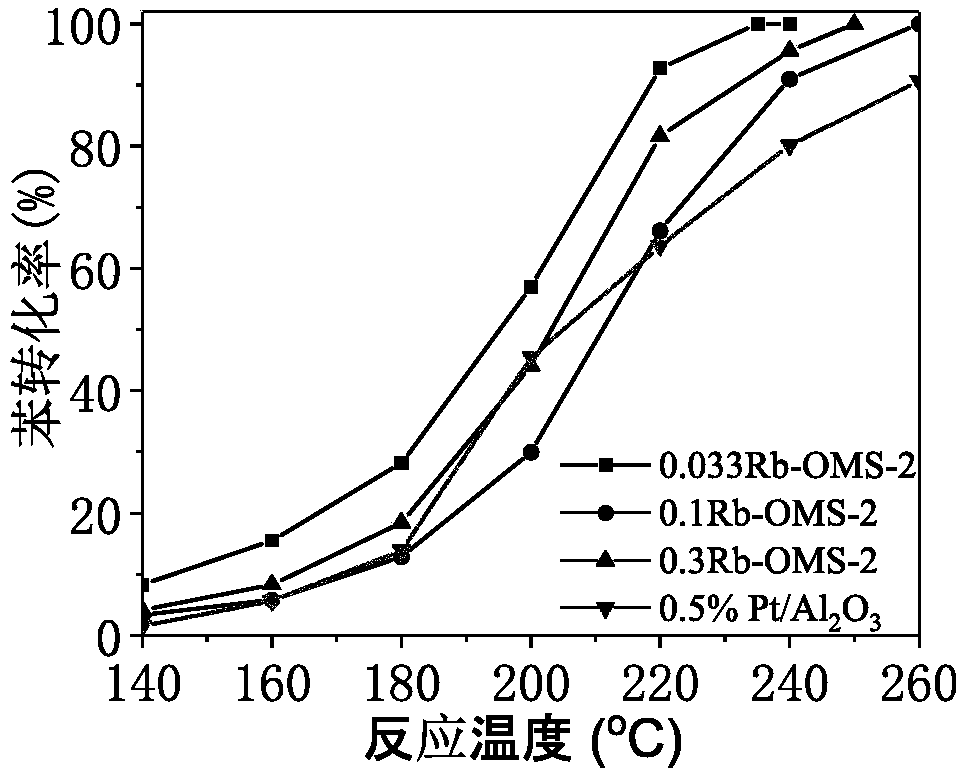
[0042] Catalyst activity evaluation: the concentration of benzene is 2000mg / m 3 , Airspeed 48000h -...
Embodiment 3
[0044] (1) The potassium permanganate that takes 0.02mmol is dissolved in the distilled water of 100mL, dissolves under magnetic stirrer;
[0045] (2) The sulfuric acid of the rubidium nitrate of 0.006mmol and the sulfuric acid of 0.5mL successively joins the solution in step (1), mixes;
[0046] (3) Add 0.01 mmol of manganese nitrate to the solution of step (2), mix well, then seal the mouth of the beaker with plastic wrap, and react at 70°C for 24 hours.
[0047] (4) After the reaction is finished, the substrate obtained by the above reaction is washed with distilled water until the conductivity is 100 μs / cm; the washed sample is dried at 50°C to obtain the 0.3Rb-OMS-2 catalyst.
[0048] Catalyst phase identification: the XRD collection of illustrative plates of the catalyst that embodiment 3 obtains is as figure 1 As shown, it is a cryptopotassium manganese ore structure.
[0049] Catalyst activity evaluation: the concentration of benzene is 2000mg / m 3 , Airspeed 48000h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com