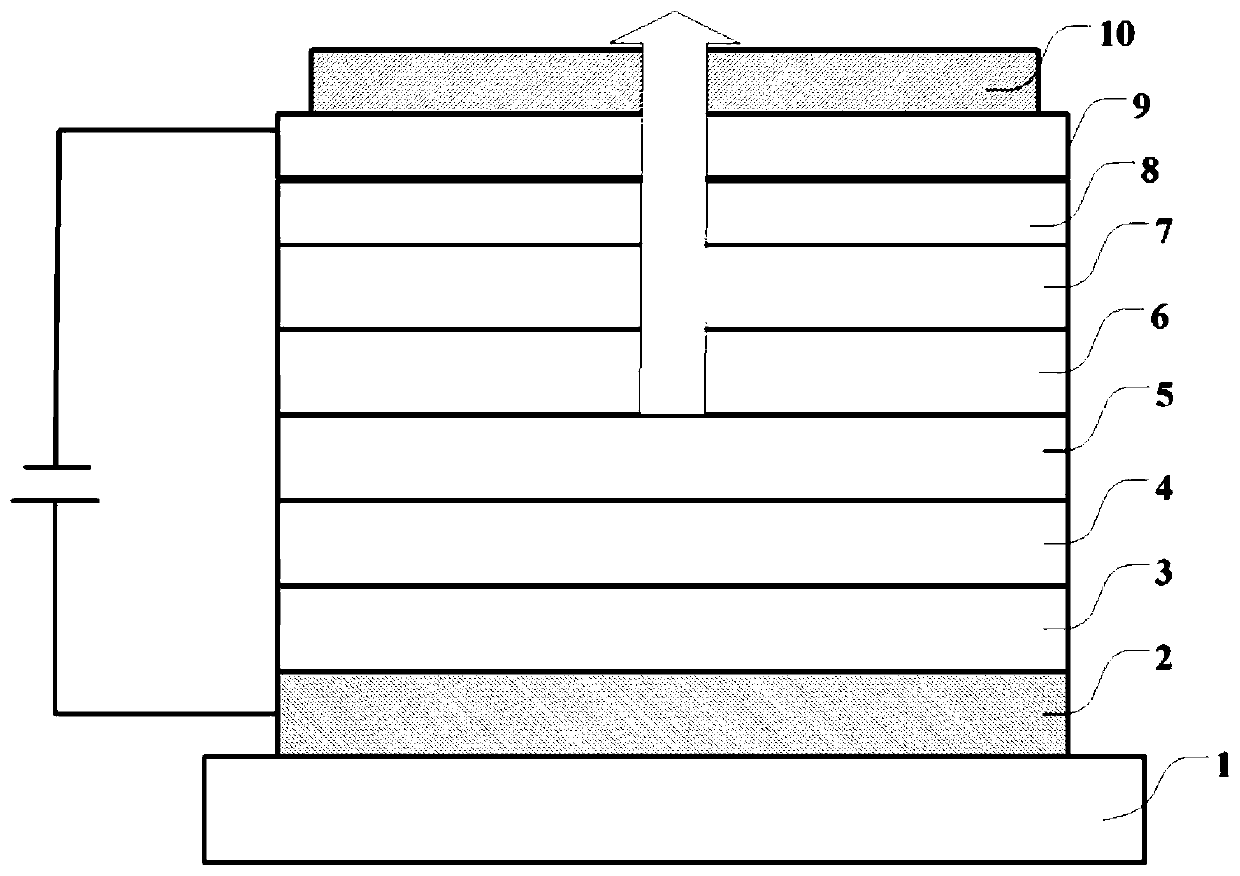
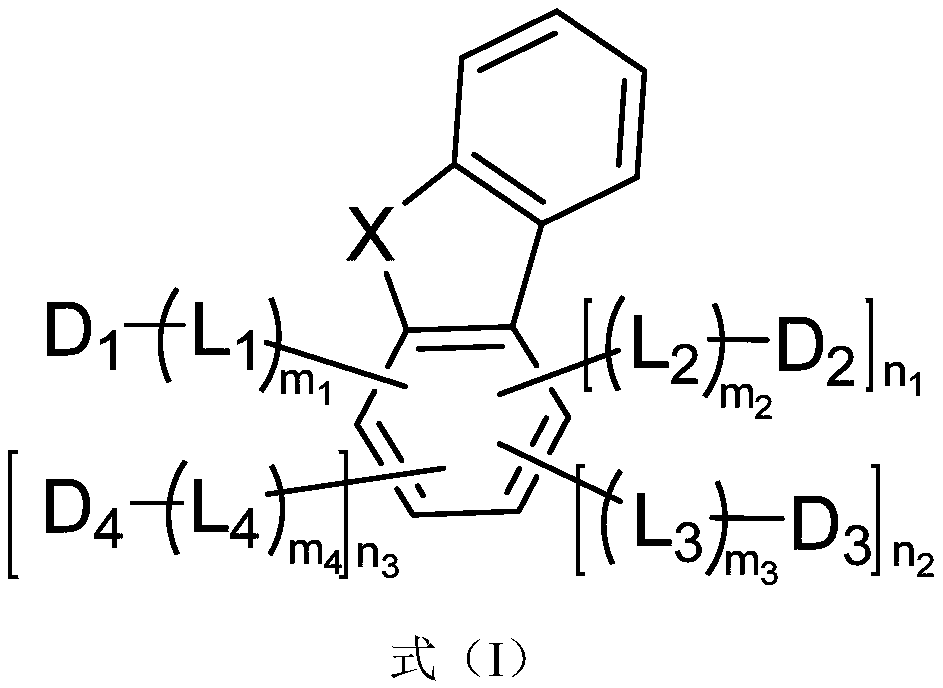
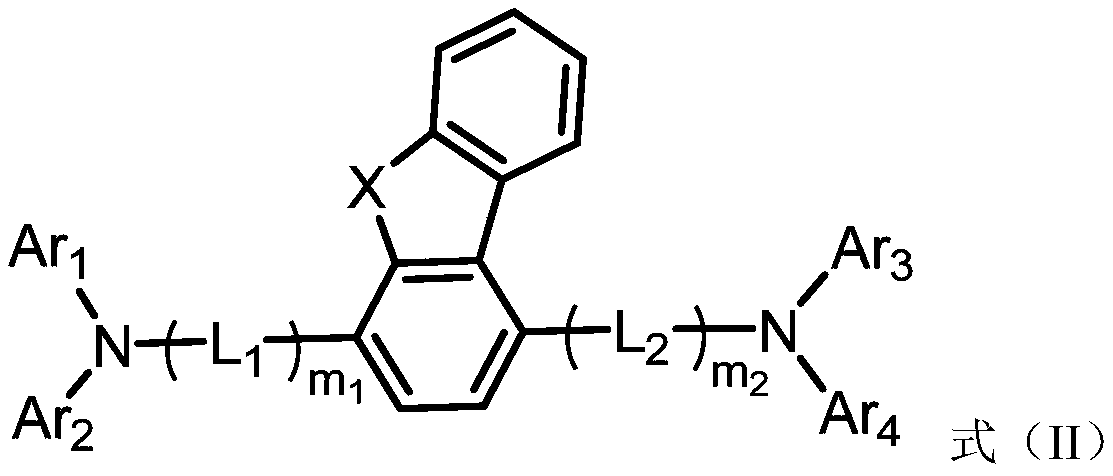
Compound, light extraction material, organic photo-electric device and electronic device
A compound and light extraction technology, applied in organic chemistry, circuits, electrical components, etc., can solve the problems of insufficient light extraction effect, insufficient high refractive index, and large difference in refractive index, etc., to improve luminous performance, High color purity, enhanced π-π stacking effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0124] Compound M1 was prepared by the following method:
[0125]
[0126] Weigh S1 (10mmol) into a 100mL two-necked flask, add 30mL nitrogen degassed toluene to dissolve S1, one of the ports is connected to a constant pressure dropping funnel, and nitrogen replaces the gas in the reaction system. Weigh NBS (10.5mmol), add 20mL of toluene to dissolve it, and add the toluene solution of NBS to the toluene solution of S1 drop by drop through the dropping funnel under the condition of 0°C and protected from light. After stirring for 2h, slowly Warm to room temperature and stir overnight. After the reaction was completed, 50 mL of deionized water was added to quench the reaction, extracted with dichloromethane (100 mL × 3), and the organic phase was collected and washed with anhydrous Na 2 SO 4 Dry processing. After filtration, the solvent was distilled off under reduced pressure with a rotary evaporator to obtain a crude product. The crude product was purified by gradient ...
preparation example 2
[0137] Compound M27 was prepared by the following method:
[0138]
[0139] Put S2 (6.5mmol), S6 (6.8mmol), tris(dibenzylideneacetone) dipalladium (0) (0.05mmol), sodium tert-butoxide (14mmol), tert-butylphosphine (0.2mmol) into 50mL three ports In the flask, while stirring, degassing and nitrogen replacement were rapidly repeated 3 times, and 20 mL of toluene was added through a syringe. The mixture was heated to reflux for 3 hours under nitrogen flow. After the reaction, water was added to the reaction solution left to cool to room temperature, extracted with dichloromethane, and washed with saturated brine. After drying the organic layer with anhydrous sodium sulfate, the solvent was distilled off and purified by column chromatography to obtain intermediate S7 (8.6 mmol, 86%).
[0140] MALDI-TOF MS: C 28 h 20 N 2 , calculated m / z: 384.2; tested: 384.5.
[0141]
[0142] Add S8 (6.2mmol), S7 (13.2mmol), tris(dibenzylideneacetone)dipalladium (0) (0.03mmol), sodium...
preparation example 3
[0146] Compound M53 was prepared by the following method:
[0147]
[0148] Add S9 (5.2mmol), S10 (5.5mmol), tris(dibenzylideneacetone)dipalladium (0) (0.05mmol), sodium tert-butoxide (10.0mmol), tert-butylphosphine (0.2mmol) into 50mL In the three-necked flask, while stirring, degassing and nitrogen replacement were repeated 3 times rapidly, and 20 mL of toluene was added through a syringe. The mixture was heated to reflux for 3 hours under nitrogen flow. After the reaction, water was added to the reaction solution left to cool to room temperature, extracted with dichloromethane, and washed with saturated brine. After drying the organic layer with anhydrous sodium sulfate, the solvent was distilled off and purified by column chromatography to obtain intermediate S11 (3.5 mmol, 67%).
[0149] MALDI-TOF MS: C 36 h 24 FNS, m / z calculated: 521.2; found: 521.5.
[0150]
[0151] Put S11 (7.8mmol), S12 (8.3mmol), tris(dibenzylideneacetone)dipalladium (0) (0.08mmol), sodi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com